| 5 |
Pyruvate dehydrogenase and the citric acid cycle |
| 5.1 |
Overview |
In the complete degradation of pyruvate, pyruvate dehydrogenase (PDH) and the citric acid cycle perform the oxidation of all substrate carbon to CO2. The hydrogen is retained in reduced form; it is subsequently oxidized in the respiratory chain.
| 5.1.1 |
Pyruvate degradation occurs in the mitochondria |
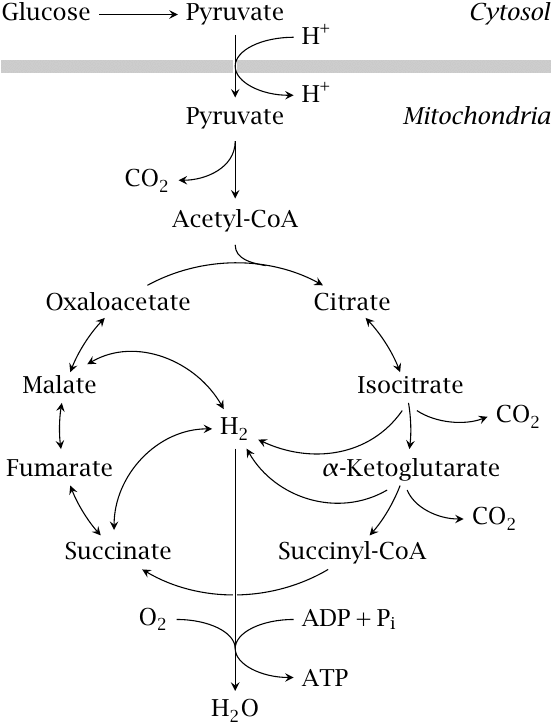
Pyruvate is produced by glycolysis in the cytosol, while PDH and all subsequent degradative steps are located in the mitochondria. Therefore, pyruvate needs to be transported from the cytosol to the mitochondrial matrix.
The outer mitochondrial membrane contains porins, which are membrane proteins that form non-specific pores and allow free permeation of most small metabolites, including pyruvate. In contrast, the inner mitochondrial membrane is much more restrictive, and it is permeable to only those metabolites for which it contains specific carrier systems. The pyruvate carrier is an active transporter that co-transports pyruvate and a proton.18
Red blood cells and blood platelets lack mitochondria and accordingly cannot degrade pyruvate. These cells reduce pyruvate to lactate, which they then release into the bloodstream (see slide 3.4.2).
| 5.2 |
Structure and function of pyruvate dehydrogenase |
Pyruvate dehydrogenase is a large and complex molecule with interesting structural, catalytic and regulatory properties.
| 5.2.1 |
The PDH reaction occurs in three successive steps that are catalyzed by three different subunits |
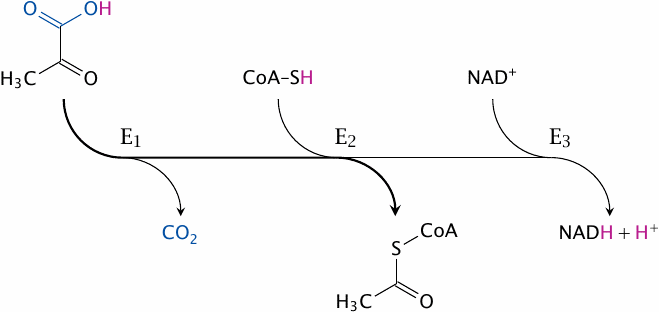
The overall reaction catalyzed by PDH is as follows:
This reaction does not occur all at once; instead, it is carried out as a sequence of group transfers and redox steps by three different catalytic subunits. These subunits are named according to the specific partial reactions they catalyze:
- 1.pyruvate dehydrogenase19 removes CO2,
- 2.dihydrolipoyl transacetylase transfers the remainder of the substrate to coenzyme A, and
- 3.dihydrolipoyl dehydrogenase transfers the H2 that was retained in the preceding steps to NAD+.
Instead of using these explicit names, we will here adopt a commonly used shorthand notation and refer to them as E1, E2, and E3, respectively.
| 5.2.2 |
The structural organization of the E. coli PDH complex |
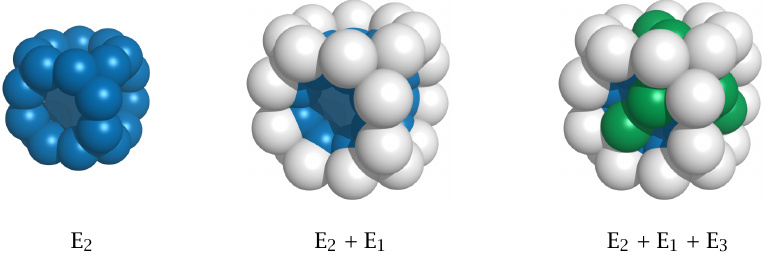
Each pyruvate dehydrogenase complex contains multiple copies of each of the three enzyme subunits. E1 and E2 are present in 24 copies each. The E. coli enzyme contains 12 copies of E3, as shown in this illustration, whereas 24 copies are found in the mammalian enzyme. In addition, the complex also contains regulatory kinase and phosphatase subunits (see slide 5.3.2).
Within this large assembly, each E1 subunit is still located close to one or more subunits of the E2 and E3 types. The reaction intermediates thus need to travel only a short distance from one active site to the next, which increases the overall catalytic efficiency. Generally speaking, high substrate throughput is the key advantage of multi-enzyme complexes over a series of individual enzymes.20
| 5.2.3 |
A lipoamide tether guides the substrate from one active site to the next |
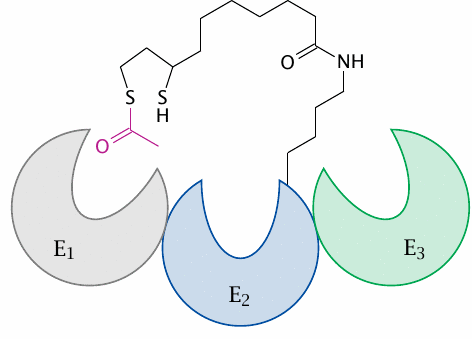
The catalytic efficiency of PDH is further increased by another neat trick: The intermediate substrates become covalently attached to lipoamide. This coenzyme is covalently attached to E2, but due to its long, flexible linker can reach into the active sites of adjacent E1 and E3 subunits as well. The lipoamide tether thus guides the substrate from one active site to the next, preventing it from leaving until it has completed the course.
| 5.2.4 |
The pyruvate dehydrogenase reaction involves multiple coenzymes |
| Coenzyme | Subunit | Role in catalysis |
| thiamine pyrophosphate | E1 | provides a carbanion for nucleophilic attack on the substrate |
| lipoamide | E2 | transfers substrate to coenzyme A, retains hydrogen |
| flavin adenine dinucleotide (FAD) | E3 | transfers H2 from lipoamide to NAD+ |
Each of the subunits E1–E3 requires a coenzyme to work its particular magic. In addition, two cosubstrates are also used, namely, NAD+ and coenzyme A.21
| 5.2.5 |
Thiamine pyrophosphate forms a carbanion |
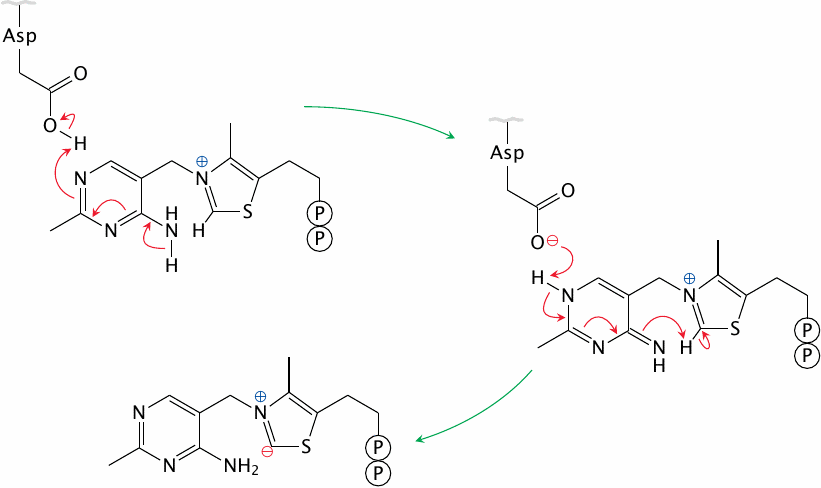
The coenzyme thiamine pyrophosphate (TPP), which is derived from thiamine (vitamin B1), is associated with the E1 subunit of PDH. In cooperation with an aspartate residue in the active site, TPP forms a carbanion, that is, a negative charge on a carbon atom. The TPP carbanion is resonance-stabilized; an electron can move back and forth between the carbanion and the neighboring cationic nitrogen.
Carbanions are very powerful nucleophiles, and the TPP carbanion functions as such in the decarboxylation of pyruvate.
| 5.2.6 |
Decarboxylation of pyruvate by E1 |
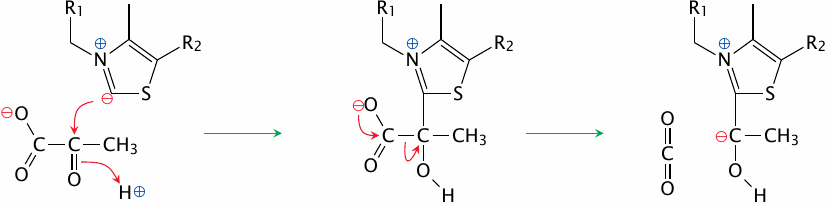
The TPP22 carbanion attacks the keto group of pyruvate, which leads to a covalent intermediate from which CO2 is cleaved. This yields another carbanion, now located within the hydroxyethyl group that is the remainder of the substrate. This new carbanion, which is again resonance-stabilized by TPP, sets the stage for the next step in the reaction.
| 5.2.7 |
Release of acetyl-CoA and disposal of hydrogen |
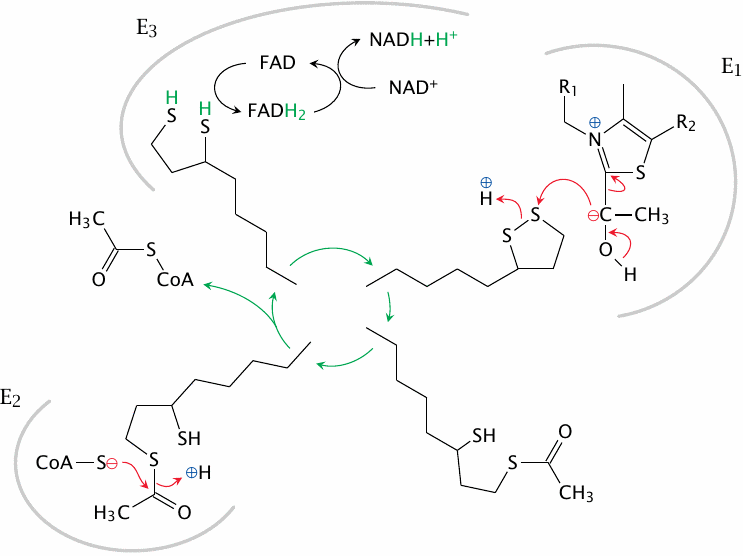
The plot continues from the previous slide at the top right. The carbanion on the hydroxyethyl group that is bound to the TPP inside E1 now attacks the disulfide bond of lipoamide. The entire substrate is then cleaved from TPP and carried off by lipoamide. Its next stop is the active site of E2, where it is transferred to coenzyme A and released as acetyl-CoA. Lipoamide is reduced to dihydrolipoamide in the process; it is reoxidized by E3, which transfers the hydrogen to NAD+, using FAD as an intermediate carrier. This concludes the pyruvate dehydrogenase reaction.
| 5.3 |
Regulation of pyruvate dehydrogenase |
We had seen earlier that enzymes may be regulated by allosteric control or through phosphorylation (section 2.5). Pyruvate dehydrogenase exemplifies both mechanisms.23
| 5.3.1 |
Alternate metabolic destinations of pyruvate |
- Conversion to acetyl-CoA by PDH for complete degradation or for synthesis of fatty acids and cholesterol
- Carboxylation to oxaloacetate, for use in gluconeogenesis or in the citric acid cycle
- Synthesis of amino acids, e.g. transamination to alanine
- Reduction to lactate
Pyruvate is used in several different pathways. All these pathways, including PDH, must be coordinated and regulated in order to achieve the proper flow rates along each of them. In the case of pyruvate dehydrogenase, the flow rate is controlled by several feedback and feed-forward mechanisms.
| 5.3.2 |
Regulation of PDH by allosteric effectors and by phosphorylation |
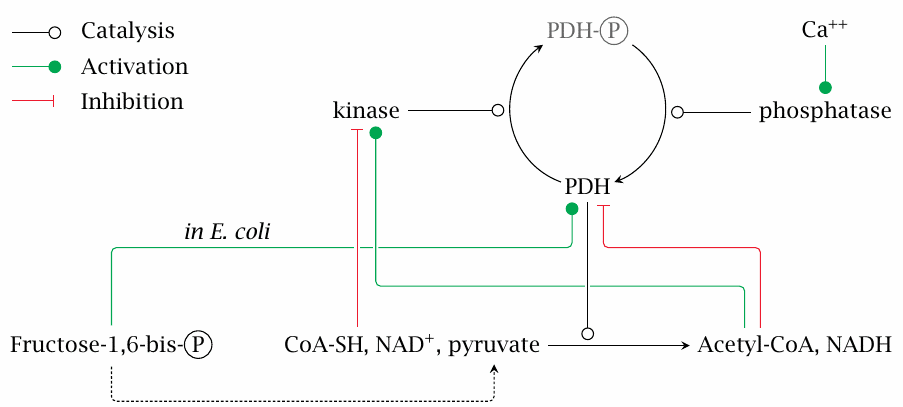
Pyruvate dehydrogenase may be allosterically activated by fructose-1,6-bisphosphate and is inhibited by NADH and acetyl-CoA.
Phosphorylation of PDH is mediated by a special regulatory enzyme, pyruvate dehydrogenase kinase. This enzyme is part of the PDH multienzyme complex. Phosphorylation inactivates pyruvate dehydrogenase. The kinase is, in turn, subject to allosteric activation by NADH and acetyl-CoA, while it is inhibited by ADP, NAD+ and by free coenzyme A. Phosphorylation is reversed, and the activity of pyruvate dehydrogenase restored by a protein phosphatase, which is also associated with the pyruvate dehydrogenase complex.
All of the above regulatory effects make good physiological sense. NADH and acetyl-CoA inhibit PDH, which means that the enzyme will slow down when its products accumulate. Such feedback inhibition is a straightforward way to link the activity of a pathway to the metabolic requirements it serves. On the other hand, pyruvate, NAD+ and, in bacteria,24 fructose-1,6-bisphosphate apply feed-forward activation—as more substrate arrives, the PDH reaction should gather speed.
One more interesting detail is that the PDH phosphatase is activated by calcium ions. Calcium ions also trigger the contraction of muscle cells. Concomitant activation of PDH anticipates the need to replace the ATP that will be consumed in the contraction.
| 5.4 |
The citric acid cycle |
Most of the carbon that accrues in carbohydrate degradation is converted by PDH to acetyl-CoA. The latter metabolite is also formed, by different enzymes, in the degradation of fatty acids and of ketogenic amino acids, and it therefore is a central hub in energy metabolism. The next step toward complete oxidation is the citric acid cycle, also referred to as the Krebs cycle or the tricarboxylic acid cycle (TCA cycle for short).
The basic idea of the TCA cycle consists in releasing the substrate carbon as CO2, while retaining the substrate hydrogen for “cold combustion” in the respiratory chain. However, a closer look reveals that something is missing from this description.
| 5.4.1 |
The overall reaction of the TCA cycle: does it add up? |
| CH3COOH | ⟶ | 2CO2+4H2 | (5.1) |
| 2H2O+CH3COOH | ⟶ | 2CO2+4H2 |
While the substrate carbon enters the TCA cycle as acetyl-CoA, the coenzyme A moiety is simply hydrolyzed off in the very first reaction; therefore, with only a little sleight of hand, we can neglect coenzyme A and substitute acetate for acetyl-CoA as the substrate.
If we look back at figure slide 5.1.1, we see that the TCA cycle produces four molecules of H2 and two molecules of CO2. Now, if we attempt to balance our single acetate substrate with these amounts of CO2 and H2, we see that we are short 4 hydrogen and 2 oxygen atoms on the left side. However, we can balance the equation by adding two molecules of water.25
What this means is that half of the H2 produced in the TCA cycle is gained by the reduction of hydrogen contained in water. The oxygen atoms that came with those two water molecules are used to complete the oxidation of the acetate carbon.26 Hydrogen derived from both water and acetate is then re-oxidized in the respiratory chain to generate ATP.
The energy yield of the TCA cycle itself, in terms of directly generated energy-rich phosphoanhydride bonds, is very modest—just one molecule of GTP, equivalent to ATP, is generated for each molecule of acetyl-CoA degraded, compared to approximately 15 ATP molecules in the respiratory chain. This comparison shows that the TCA cycle’s main contribution to ATP generation is to provide H2 for the respiratory chain.
| 5.4.2 |
The citrate synthase reaction |
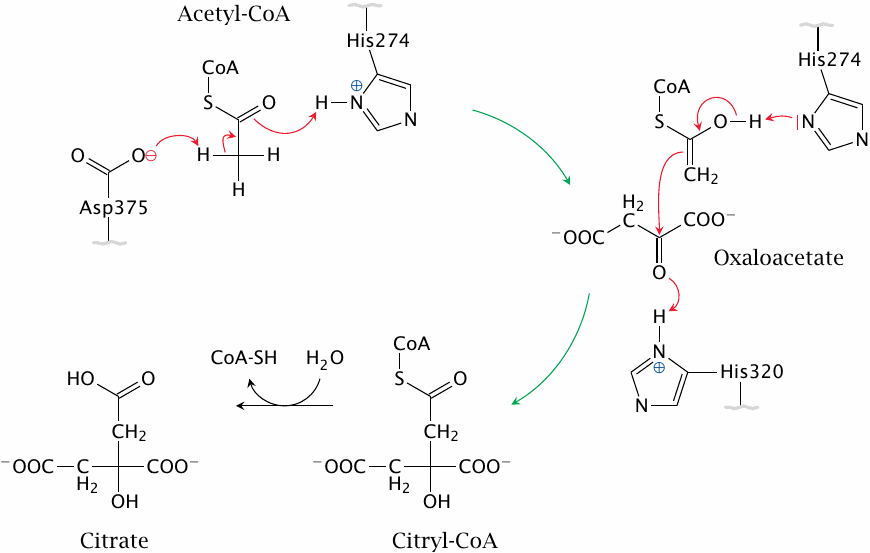
The first reaction in the TCA cycle is catalyzed by citrate synthase. It is mediated by acid-base catalysis; abstraction of a proton from the methyl group of acetyl-CoA by an aspartate residue in the active site converts acetyl-CoA to an enol form, which then attacks the carbonyl group of oxaloacetate. The reaction is assisted by two histidine residues and pulled forward by the subsequent hydrolysis of citryl-CoA. Figure drawn after a scheme given in [18].
| 5.4.3 |
Reactions in the TCA cycle: from citrate to oxaloacetate |
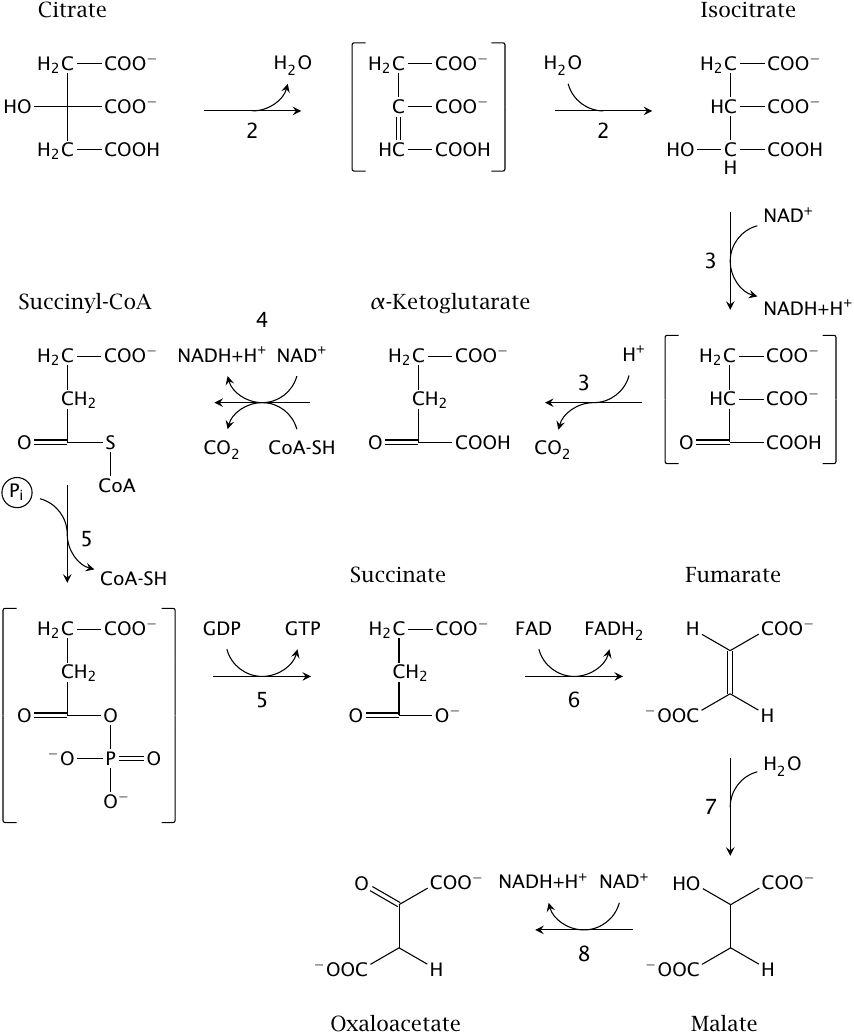
The paragraph numbers below correspond to those of the reactions in the figure. The first reaction in the figure is the second in the cycle overall, which is why it gets the number 2.
- 2.The hydroxyl group in the newly formed citrate is shifted to an adjacent carbon to yield isocitrate. This reaction is catalyzed by citrate isomerase and involves the transient elimination of water across the two carbons involved; the water is then added back in the reverse orientation.
- 3.Isocitrate is decarboxylated and dehydrogenated to α-ketoglutarate by isocitrate dehydrogenase. In contrast to the pyruvate dehydrogenase reaction, dehydrogenation here precedes decarboxylation. The dehydrogenated intermediate is known as oxalosuccinate.
- 4.α-Ketoglutarate is converted to succinyl-CoA by α-ketoglutarate dehydrogenase. This catalytic mechanism of this enzyme is completely analogous to that of pyruvate dehydrogenase.
- 5.Succinyl-CoA is converted to succinate by succinate thiokinase, and GDP is concomitantly phosphorylated to GTP. From the mechanism of the glyceraldehyde-3-phosphate dehydrogenase reaction (Figure 3.3.5), we already know that thioester bonds are energy-rich and can drive the phosphorylation of carboxylic acids. A carboxylic acid phosphate, succinylphosphate, also occurs as an intermediate in the succinate thiokinase reaction. As is the case with 1,3-bisphosphoglycerate, the phosphate group is subsequently transferred to a nucleotide diphosphate. While succinate thiokinase uses GDP rather than ADP, the amounts of energy involved are virtually the same.
- 6.Succinate is dehydrogenated across the CH2–CH2 bond by succinate dehydrogenase, which yields fumarate. The coenzyme used in this reaction is flavin adenine dinucleotide (FAD). As a rule of thumb, you can assume that FAD is used in the dehydrogenation of CH–CH bonds, whereas either NAD+ or NADP+ are used in the dehydrogenation of CH–OH bonds. While all other enzymes in the TCA are in aqueous solution in the mitochondrial matrix, succinate dehydrogenase is bound to the inner surface of the inner mitochondrial membrane; it is identical with complex II of the respiratory chain (see slide 6.4).
- 7.Fumarate is hydrated to l-malate by fumarase.
- 8.Malate is dehydrogenated by malate dehydrogenase to oxaloacetate. This step regenerates oxaloacetate, which can again enter the citrate synthase reaction, and thus completes the cycle.
| 5.4.4 |
α-Ketoglutarate dehydrogenase resembles PDH |
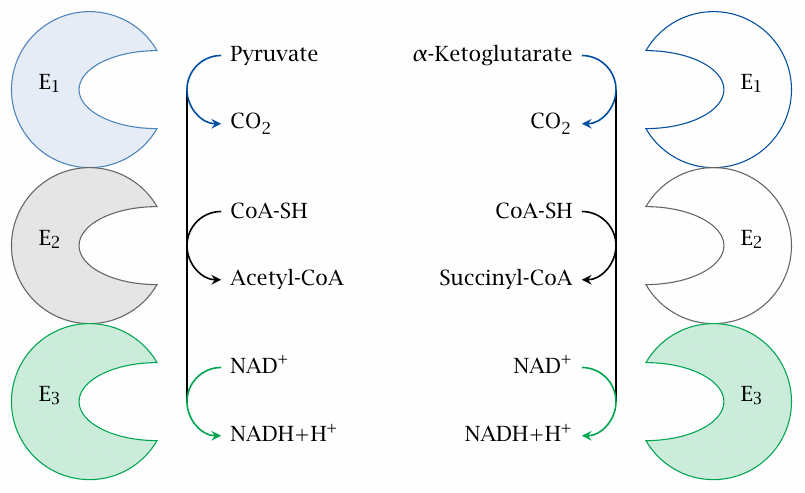
As pointed out above, α-ketoglutarate dehydrogenase uses the same catalytic mechanism as pyruvate dehydrogenase. The similarity is reflected in a high degree of homology between the subunits of the two enzymes. If you look closely at the PDH mechanism (Figure 5.2.1), you will see that the reactions carried out by the final subunit (E3) will be identical in both cases, since E3 comes into play when only hydrogen is left but the rest of the substrate has already been disposed of. Indeed, the two multienzyme complexes share the very same E3 protein; only E1 and E2 are specific for the respective substrates. The same E3 subunit occurs yet again in an analogous multienzyme complex that participates in the degradation of the branched chain amino acids (slide 12.4.4), as well as in the glycine cleavage system (slide 15.2.6).
| 5.5 |
Regulation of the citric acid cycle |
- ATP and NADH inhibit isocitrate dehydrogenase
- NADH inhibits α-ketoglutarate dehydrogenase
- High levels of NADH will lower the oxaloacetate concentration, which limits citrate synthase activity
Acetyl-CoA is not only utilized for complete oxidation but also for the biosynthesis of fatty acids, cholesterol, and ketone bodies. Therefore, the activity of the citric acid cycle must be balanced with those of the various synthetic pathways. This regulation is mainly exercised by NADH, the major direct product of the TCA, and by ATP, the ultimate product of complete substrate oxidation via the TCA and the respiratory chain.
It is noteworthy that the equilibrium of the malate dehydrogenase reaction favors malate. The concentration of oxaloacetate is thus quite low, and it will be lowered further if NADH accumulates. This limits the rate of the initial reaction of the TCA, that is, the synthesis of citrate, and it also detracts from the free energy of that reaction. To make citrate synthesis go forward, it is necessary to sacrifice the energy-rich thioester bond in the citryl-CoA intermediate, which in contrast to succinyl-CoA is simply hydrolyzed and not used toward the synthesis of GTP or ATP.
The mitochondrion contains two isozymes of isocitrate dehydrogenase; one uses NAD+ and the other NADP+ as the cosubstrate. Intriguingly, the feedback inhibition by NADH and ATP applies only to the NAD+-dependent isozyme, but not to the NADP+-dependent one.
In cells with high TCA activity, such as heart and skeletal muscle, the NADP-dependent variant is actually present at higher activity than the NAD-dependent one. How, then, is the NADP-dependent enzyme regulated? This regulation appears to occur in coordination with the flow through the respiratory chain and the proton-motive at the inner mitochondrial membrane. The mechanism is quite fascinating and is discussed at the end of the following chapter.
| 5.6 |
Reactions that divert and replenish TCA cycle intermediates |
Several metabolites in the citric acid cycle are also substrates in biosynthetic pathways, for example those for heme or various amino acids, and through these pathways are drained from the cycle. When this occurs, they will need to be replenished. Similarly, when the workload of a cell and its ATP demand increase, the TCA cycle must then process acetyl-CoA at an accelerated rate, which requires an increase in the pool of TCA cycle intermediates.
The first thing to note is that just feeding more acetyl-CoA into the TCA cycle does not address this problem, since acetyl-CoA simply offsets the two CO2 molecules that are lost in subsequent reactions in the cycle. Instead, we need a net supply of any of the intermediates with four or more carbon atoms that function catalytically rather than as substrates.
One important and abundant source of TCA cycle intermediates is the pyruvate carboxylase reaction, which makes oxaloacetate from pyruvate (slide 7.2.4). Often, however, the oxaloacetate thus obtained is immediately diverted again toward glucose synthesis (gluconeogenesis). In this situation, amino acids become the major net source of TCA cycle intermediates (see chapter 12).