| 10 |
Triacylglycerol metabolism |
| 10.1 |
Overview |
Various types of lipids occur in the human body, namely 1)triacylglycerol, 2)cholesterol, and 3)polar lipids, which include phospholipids, glycolipids and sphingolipids. This chapter will focus on triacylglycerol; cholesterol will be covered in a separate chapter. The metabolism of polar lipids will not be covered systematically.
In contrast to polar lipids and cholesterol, which are found in the membranes of every cell, triacylglycerol is concentrated mostly in adipose (fat) tissue; minor amounts of triacylglycerol occur in other cell types, such as liver epithelia and skeletal muscle fibers. Yet, overall, triacylglycerol is the most abundant lipid species, and the only one with an important role in energy metabolism.
Triacylglycerol occurs in human metabolism in two roles, namely (a)as a foodstuff, which accounts for a significant fraction of our caloric intake, and (b)as a store of metabolic energy. This store can be replenished using dietary triacylglycerol or through endogenous synthesis from carbohydrates or proteins.
| 10.1.1 |
Foodstuffs and their energy contents |
| Foodstuff | Energy (kcal/g) |
| protein | 4 |
| carbohydrates | 4 |
| triacylglycerol | 9 |
| alcohol | 7 |
The amount of energy stored per gram of tissue is far higher in fat than in any other tissue, for two reasons:
- 1.One gram of triacylglycerol itself contains more than twice as many calories as one gram of carbohydrates or protein. This is simply because triacylglycerol contains much less oxygen than carbohydrates, in which oxygen contributes half the mass but essentially no metabolic energy. Similarly, the oxygen, nitrogen and sulfur contained in protein detract from its energy density.
- 2.Triacylglycerol in fat cells coalesces to droplets that are entirely free of water. In contrast, protein and carbohydrates, including glycogen, always remain hydrated, which further diminishes the density of energy storage.
| 10.1.2 |
Carbon pools in carbohydrate and fat metabolism |
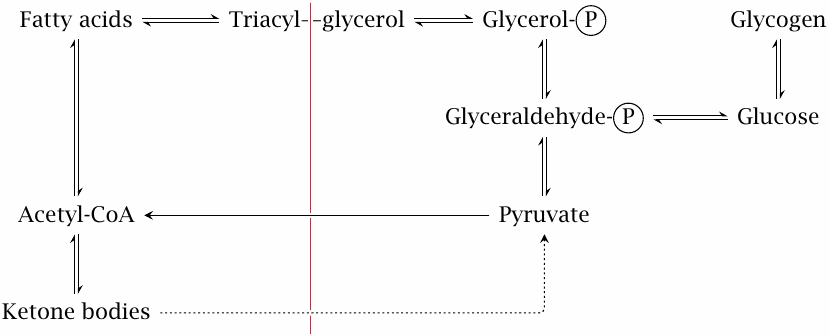
Because of its high energy density, it makes sense that fat forms the largest store of energy, while only a comparatively small amount is stored as glycogen. There is, however, one limitation to the usefulness of triacylglycerol: the conversion of carbohydrate or protein to fatty acids via acetyl-CoA is more or less a one-way street. Carbon stored as triacylglycerol is, for the most part, confined to the limited scope of pathways on the left of the red line in this slide. Within this pool, the carbon may be turned into free fatty acids, which can serve as fuel for heart and skeletal muscle, or into ketone bodies, which can supply energy to several more organs, including the brain. However, it can no longer be turned back into glucose; or at least not efficiently so, since the dotted “bootlegger’s pathway” that leads from ketone bodies to pyruvate (and from there again to glucose) has limited capacity (see slide 10.4.3).
Because of the restricted metabolic scope of triacylglycerol, we cannot rely exclusively on fat degradation in a catabolic situation. Once glycogen is used up, continued fasting will deplete not only stored fat but also protein, most notably in skeletal muscle, which is necessary to maintain a sufficient supply of glucose via gluconeogenesis.
| 10.2 |
Digestion and uptake of dietary triacylglycerol |
As discussed in the first chapter (slide 1.6.8), dietary triacylglycerol undergoes hydrolysis in the digestive tract. The main products of hydrolysis are monoacylglycerol and free fatty acids.
| 10.2.1 |
Triacylglycerol and its cleavage products |
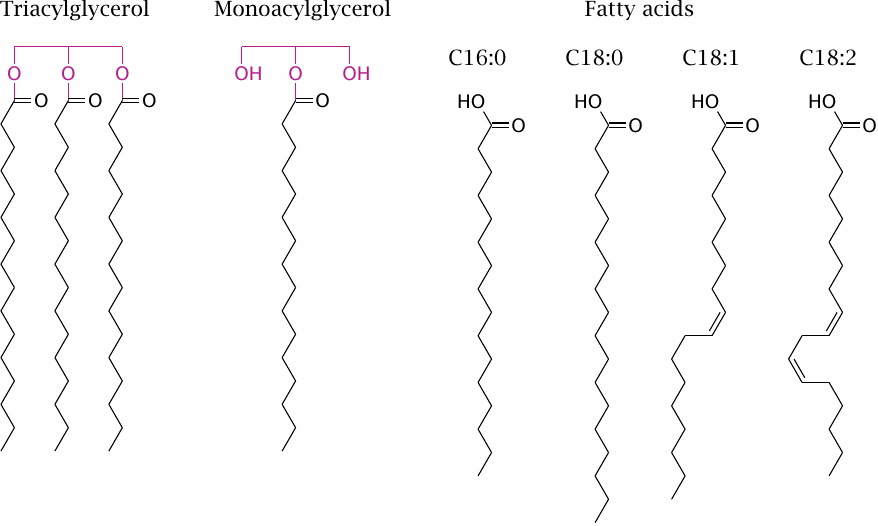
The fatty acids found in natural fats vary both in chain length and in the number of double bonds. The slide shows the four most abundant species; from the left to the right, their trivial names are palmitate, stearate, oleate, and linoleate. Other chain lengths and degrees of unsaturation occur.56 A minor fraction of dietary fatty acids, mostly from plants, contain less than 12 carbon atoms; these so-called medium-chain fatty acids have some peculiar metabolic properties that make them therapeutically useful in certain diseases (slide 10.2.7).
| 10.2.2 |
Solubilization of fat by detergents |
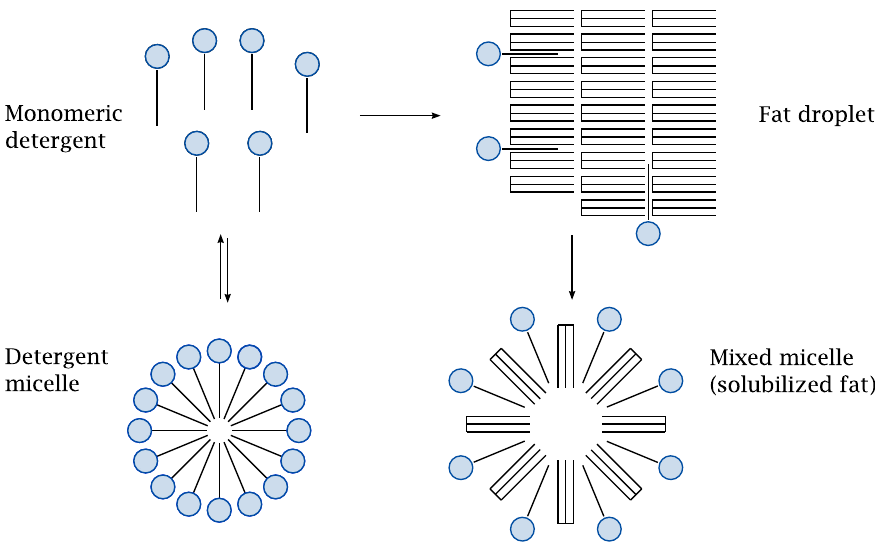
Detergents are amphiphilic molecules that are freely soluble in water at low concentrations but reversibly aggregate into micelles at higher ones. When mixed with fat, monomeric detergent molecules penetrate the fat particles and break them up into mixed micelles; in this form, the fat becomes amenable to cleavage by lipases. The fatty acids released by enzymatic hydrolysis act as detergents themselves and will aid in the solubilization of remaining fat during digestion.57
The critical micellar concentration (CMC) of a detergent is the threshold above which any additional detergent will coalesce into micelles. Since only the monomeric detergent molecules can interact with fat, it follows that detergents with higher CMC values will solubilize fat more rapidly. Bile acids have particularly high CMC values and solubilize fat very swiftly.58
While most of the dietary triacylglycerol is hydrolyzed by pancreatic triacylglycerol lipase in the small intestine, fat digestion is already initiated by gastric lipase, which is released by the mucous membrane of the stomach [51]. In vitro, this enzyme can be activated by bile acids also, but since these are absent from the stomach, the question arises what molecules might serve as auxiliary solubilizing agents for gastric lipase in vivo. Plausible candidates are dietary proteins and phospholipids [52].
| 10.2.3 |
Uptake and re-packaging of digested fat in the small intestine |
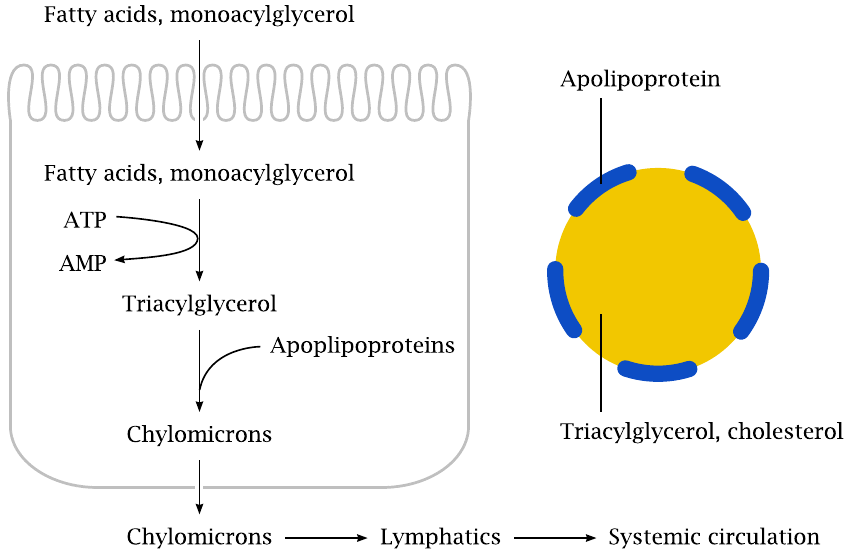
After solubilization and lipase digestion, monoacylglycerol and free fatty acids are taken up by epithelial cells in the mucous membrane of the small intestine. What happens to them once inside is somewhat surprising: they are immediately converted back to triacylglycerol. This involves the transient activation of fatty acids to acyl-CoA (see slide 10.3.1) at the expense of ATP.59 The newly formed fat is then combined with protein molecules called apolipoproteins into lipoprotein particles, such that the proteins form a hydrophilic shell around the lipid core. Some phospholipids are included as well and complete the hydrophilic shell.
Lipoproteins occur in various subtypes (see slide 11.4.2). The specific type formed at this stage, the chylomicrons, are the largest of all lipoproteins, with a molecular mass of up to 1010 Dalton, a diameter up to 1 µm, and approximately 107 molecules of triacylglycerol. The chylomicrons also transport dietary cholesterol; this is discussed in slide 11.4.3.
Like glucose and other solutes taken up from the gut, the chylomicrons are released into the extracellular space at the basolateral side of the intestinal epithelia. However, unlike those solutes, the chylomicrons are not drained toward the liver via the portal vein, but instead are drained via the lymphatics. This is explained in the next two slides.
| 10.2.4 |
The lymphatics drain excess fluid from the interstitial space |
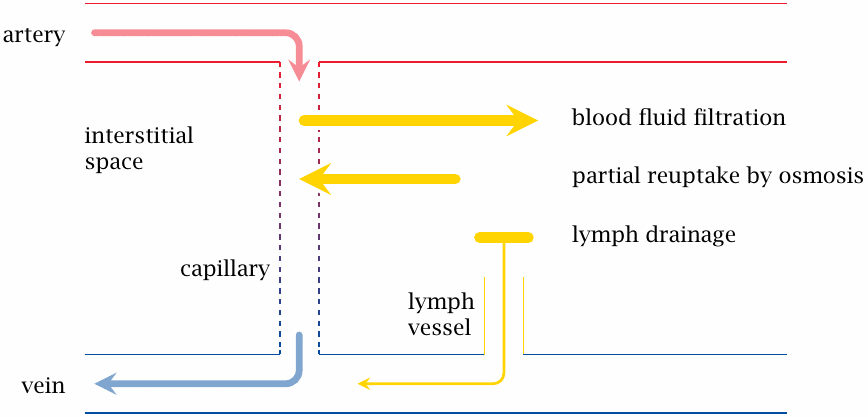
This slide and the next one introduce a bit of background to explain how chylomicrons are transported from the intestine to the systemic circulation.
The capillaries of the blood circulation are porous, and the hydrostatic pressure within them drives the filtration of plasma fluid into the interstitial space. Since the pores in the capillary walls are small, filtration is limited to water and small solutes. Albumin and other plasma proteins are not filtrated and therefore maintain an osmotic pressure gradient that opposes and mostly compensates for hydrostatic filtration. The fraction of the filtrate that is not recovered through osmosis is drained by lymphatic vessels and then back to the venous side of the systemic circulation.60
| 10.2.5 |
Chylomicrons are drained from the intestine through the lymphatics, bypassing the liver |
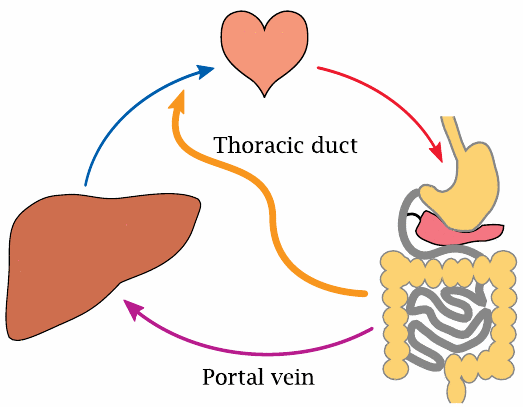
Just as plasma proteins are excluded from diffusing out of capillaries, the chylomicrons are excluded from diffusing into them. Chylomicrons thus cannot enter the circulation directly and must instead be drained through the lymphatic system. The thoracic duct, which is the major effluent of the entire lymphatic system, joins one of the major veins just a short distance upstream of the heart, but downstream of the liver. Therefore, unlike glucose and other small molecules that are taken up in the intestines, chylomicrons bypass the portal circulation and the liver. They will, however, reach the liver via the systemic circulation at a later stage (see next slide).
| 10.2.6 |
Lipoprotein lipase extracts triacylglycerol from chylomicrons |
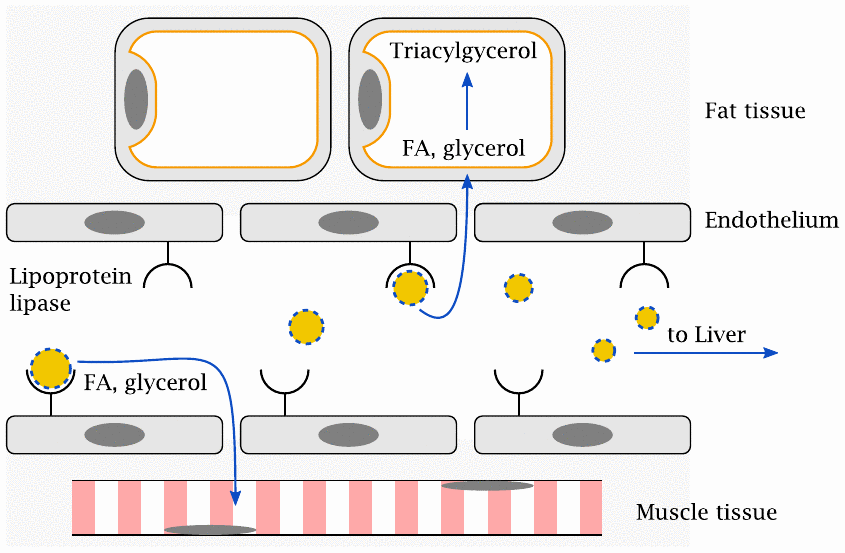
Once the chylomicrons have entered the circulation, the capillary wall barrier must again be overcome in the delivery of triacylglycerol to extravascular cells. This is accomplished with the help of lipoprotein lipase, which is located on the endothelial surface. It binds the chylomicrons and extracts triacylglycerol from them, which it then cleaves again to fatty acids and glycerol. These small molecules can cross the endothelial barrier by diffusion and reach the cells in the surrounding tissue.
In adipose cells, the fatty acids are combined with glycerol yet again for storage. In other cell types, most notably muscle cells, they may either be stored or degraded directly to acetyl-CoA, which is then consumed in the TCA cycle and the respiratory chain. The remnants of chylomicrons, depleted of most of their triacylglycerol, are captured by the liver, endocytosed, and degraded. The cholesterol and remaining fat released in the process is either utilized in the liver or repackaged into other lipoprotein particles.61
| 10.2.7 |
Medium-chain fatty acids |
- contain less than 12 carbon atoms
- low content in most foods, but relatively high (10–15%) in palm seed and coconut oil, from which they are industrially prepared
- triglycerides with medium chains are more soluble and more rapidly hydrolyzed by gastric and pancreatic lipase
- not efficiently re-esterified inside intestinal cells; systemic uptake mostly as free fatty acids
- reach mitochondria by diffusion, without prior activation to acyl-CoA and acyl-carnitine
Medium-chain fatty acids (MCFA) are not a major constituent of our regular diet, nor are they required; however, they can be useful in patients whose ability to digest or absorb regular fats is compromised. They are more readily hydrolyzed by enzymes, in particular by gastric lipase, which usually plays a minor role only in the digestion of triacylglycerol with regular, longer acyl chains.
Gastric lipase continues to be secreted, even at increased levels [53], when pancreatic lipase is lacking due to exocrine pancreas insufficiency. Therefore, MCFA triglycerides can still be processed in this situation. They are used in the dietary treatment of such patients and also of those with other types of fat maldigestion and malabsorption.
| 10.3 |
Utilization of fatty acids: β-oxidation |
As briefly mentioned before, the utilization of fatty acids occurs by way of conversion to acetyl-CoA, which is accomplished in β-oxidation. This pathway runs in the mitochondria, so the first task after cellular uptake of the fatty acid molecule is to get it into the mitochondrion.
| 10.3.1 |
Two activated forms of fatty acids |
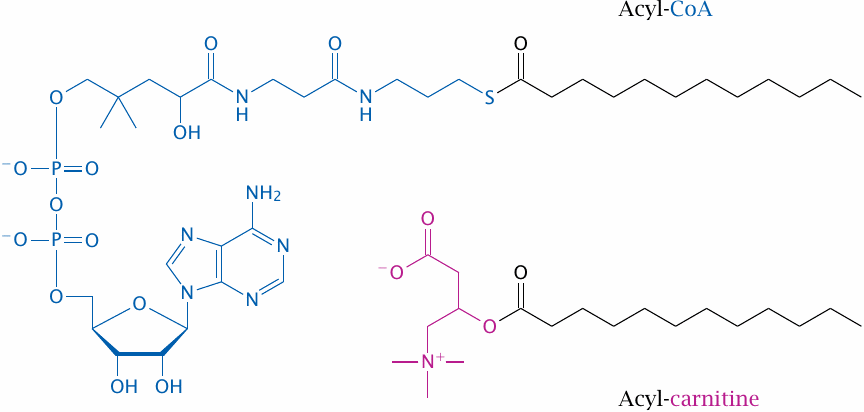
Fatty acids are initially activated to fatty acyl-CoA in the cytosol. This is also the form in which they enter degradation by β-oxidation. However, during transport, the CoA-moiety is transiently replaced by carnitine. This slide shows the structures of both the CoA- and the carnitine-activated forms; the entire transport process is outlined in the next one.
| 10.3.2 |
Activation of fatty acids and transport to the mitochondrion |
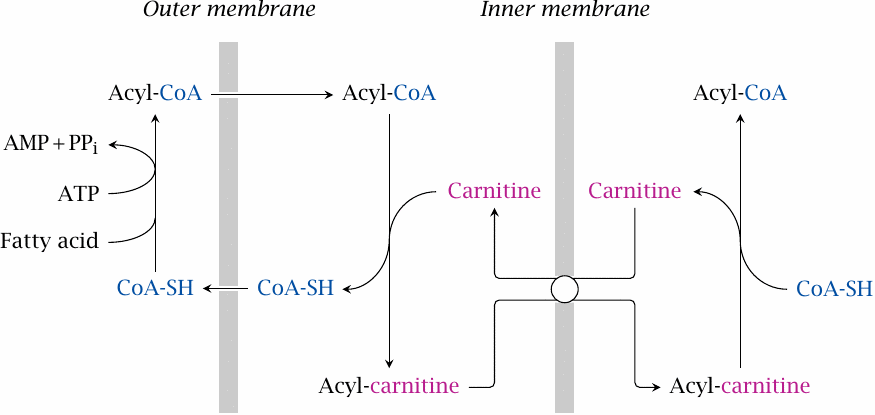
Fatty acids are activated in the cytosol to acyl-CoA by acyl thiokinase, also known as acyl-CoA synthetase. After transport across the outer mitochondrial membrane, the acyl group is transferred to carnitine by carnitine acyltransferase. Exchange for free carnitine transports the acylcarnitine molecule into the mitochondrial matrix, where carnitine is replaced again with coenzyme A by a second acyl transferase.
One unusual aspect of this transport process is that the energy of the thioester bond in acyl-CoA seems to be sufficiently well preserved in the ester bond of acylcarnitine to allow the exchange reaction to be reversed inside the mitochondrion. Carboxyl esters bonds don’t usually have a sufficiently high energy content for that. I once stumbled upon a theoretical paper explaining why carnitine is special in this regard, but it went straight over my head, and I therefore cannot give you an explanation.
| 10.3.3 |
Reactions in β-oxidation |
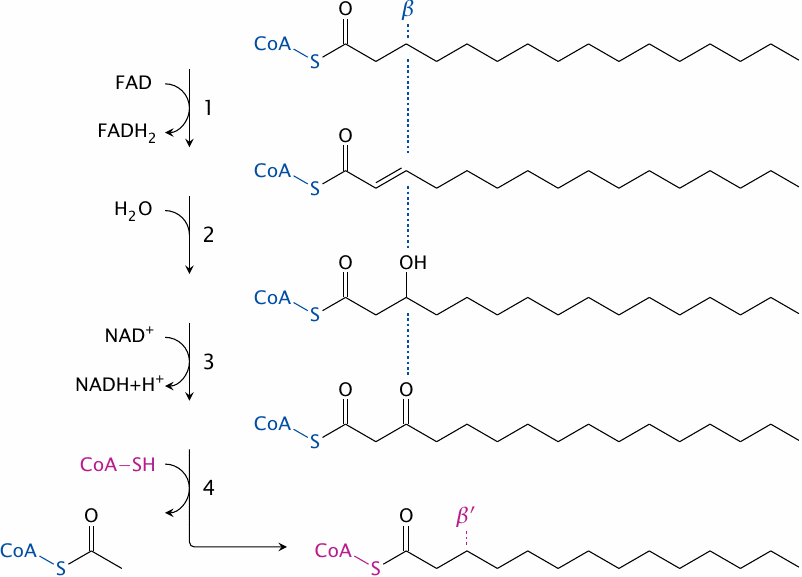
The term β-oxidation refers to the Greek lettering of the carbons in organic molecules: the α carbon is right beside a functional group, and the β carbon is the next one. It is at the second carbon from the thioester, then, where the action is in β-oxidation. The reactions are as follows:
- 1.The fatty acyl-CoA molecule is first dehydrogenated between the α and the β carbon atoms by acyl-CoA dehydrogenase. FAD accepts the hydrogen abstracted and is reduced to FADH2. This yields 2-trans-enoyl-CoA.
- 2.The trans double bond just created is hydrated—that is, water is added to it—by enoyl-CoA hydratase, which yields hydroxyacyl-CoA. The α carbon is now once more fully reduced.
- 3.The β-hydroxyl group is converted to a keto group by hydroxyacyl-CoA dehydrogenase. NAD+ accepts the hydrogen. The product is β-ketoacyl-CoA.
- 4.Thiolase introduces a new molecule of coenzyme A to cleave the β-ketoacyl-CoA, which releases acetyl-CoA and a new, shortened acyl-CoA that enters the next cycle of β-oxidation.
The process is repeated until the fatty acid is completely broken down. In the case of acyl chains with even numbers of carbons, this will yield acetyl-CoA only, whereas those with odd numbers of carbons will yield one molecule of propionyl-CoA in the final thiolase step. There is a special pathway to take care of the propionyl-CoA, which is surprisingly complicated (see slide 10.3.6).
| 10.3.5 |
The reaction mechanism of thiolase |
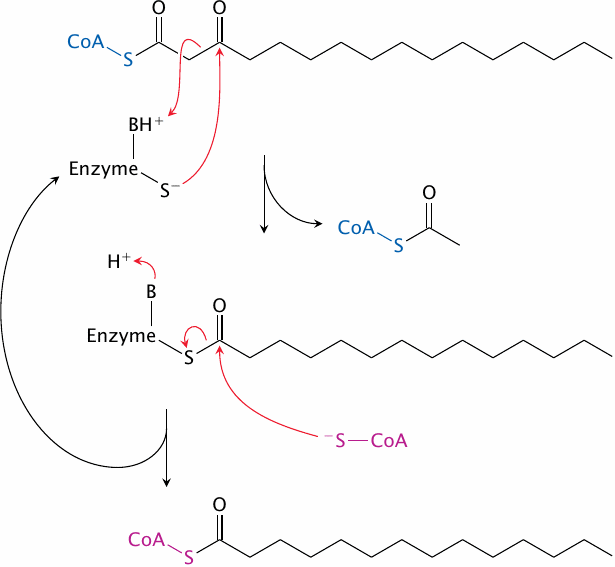
The thiolase mechanism does not have a closely analogous precedent among the reactions we have seen so far. However, if we look at the individual steps of the thiolase reaction, we can still recognize some familiar features:
- 1.The nucleophilic attack of a cysteine thiolate in the active site on a carbonyl group in the substrate yields a covalent intermediate. This also happens with glyceraldehyde-3-phosphate dehydrogenase (see slide 3.3.5).
- 2.Acid-base catalysis breaks a C–C bond adjacent to the carbonyl bond. Note that the thiolase reaction is reversible. Making of a C–C bond adjacent to the carbonyl group by acid-base catalysis then occurs in the reverse reaction, which we have seen before with citrate synthase (slide 5.4.2).
- 3.The creation of one thioester at the expense of another occurs in the second step of the pyruvate dehydrogenase reaction (see slide 5.2.7).
| 10.3.6 |
Utilization of propionate |
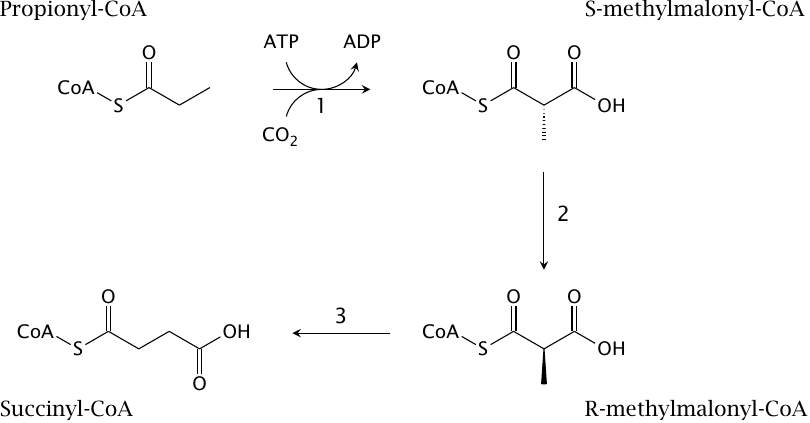
Fatty acids with odd numbers of carbon atoms yield one molecule of propionyl-CoA as the final degradation product. This metabolite has a rather elaborate degradative pathway:
- 1.Initially, propionyl-CoA is converted to S-methylmalonyl-CoA by propionyl-CoA carboxylase. This reaction uses CO2 and ATP, with biotin serving as a coenzyme; the mechanism resembles that of pyruvate carboxylase (slide 7.2.4).
- 2.S-methylmalonyl-CoA is converted to its enantiomer (R-methylmalonyl-CoA) by methylmalonyl-CoA racemase.
- 3.Finally, one carboxyl group is transplanted within the molecule by methylmalonyl-CoA mutase to yield succinyl-CoA. This is a frighteningly complex reaction; we will skip the gory details and just note that it requires vitamin B12, in the form of adenosylcobalamin, as a coenzyme. When this vitamin is lacking, methylmalonyl-CoA backs up and is hydrolyzed to free methylmalonate, which may inhibit gluconeogenesis (see slide 7.4.1).
Note that succinyl-CoA is a citric acid cycle intermediate. It can therefore enter gluconeogenesis (see slide 7.1.1), so that propionyl-CoA is an exception to the rule that carbon from fatty acids cannot be used for gluconeogenesis. However, since fatty acids with odd numbers of carbons amount to a small fraction of all fatty acids, this exception is not quantitatively very important.
| 10.3.7 |
Organ relationships in triacylglycerol utilization |
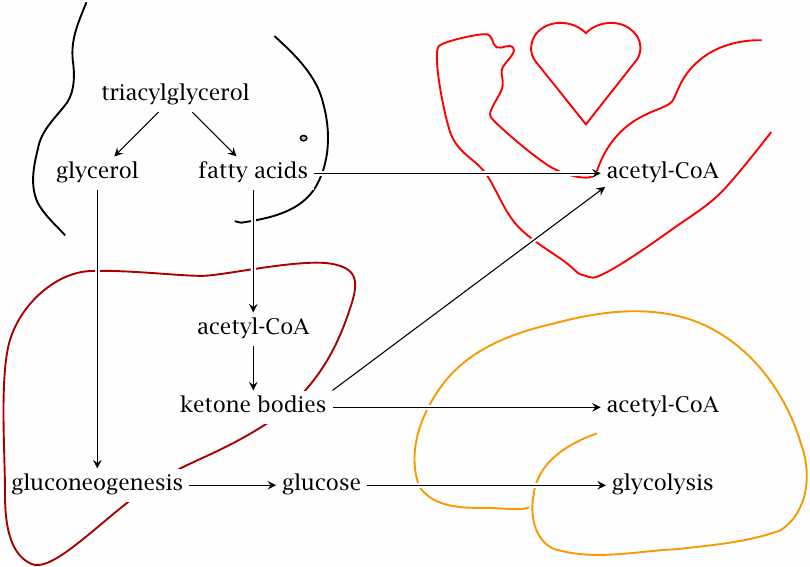
When triacylglycerol is mobilized from the fat tissue, its hydrolysis to free fatty acids and glycerol is initiated by an enzyme named hormone-sensitive lipase. This lipase is stimulated downstream of glucagon and epinephrine, the antagonist hormones of insulin. As seen before in carbohydrate metabolism (slides 7.5.4 and 8.4.2), the two hormones activate adenylate cyclase and protein kinase A. The fatty acids produced by the lipase are released into the bloodstream, where they bind to albumin for transport. The fatty acids can be utilized in two different ways:
- 1.Directly, that is, they are taken up by the energy-requiring target tissue and degraded by β-oxidation. We noted above that β-oxidation is preceded by the translocation to the mitochondrion, which requires carnitine. About 95% of all carnitine is found in skeletal muscle,63 which implies that muscle tissue is the major client for direct utilization. The heart muscle cells also consume fatty acids through β-oxidation.
- 2.Indirectly, after initial conversion to ketone bodies in the liver. This term comprises two small organic acids, acetoacetate and β-hydroxybutyrate, as well as acetone. The brain, which in the well-fed state only utilizes glucose for ATP regeneration, is able to replace up to 50% of its usual glucose consumption with ketone bodies during starvation. The brain is responsible for approximately 20% of our total energy consumption at rest, and therefore is a major consumer of ketone bodies. Other major clients are the heart and skeletal muscle.
The second cleavage product of fat, glycerol, is released into the bloodstream and picked up by the liver as well. It is phosphorylated by glycerol kinase to glycerophosphate. As we have seen before (slide 6.9.3), this metabolite can be dehydrogenated to dihydroxyacetone phosphate, which may enter gluconeogenesis. Glycerol released from fat constitutes a minor source of glucose in times of starvation.
The pattern of fat usage sketched out here holds for the bulk of all adipose tissue, which is white. An exception from this pattern is brown fat tissue, which is covered in the next slide.
| 10.3.8 |
Brown fat tissue |
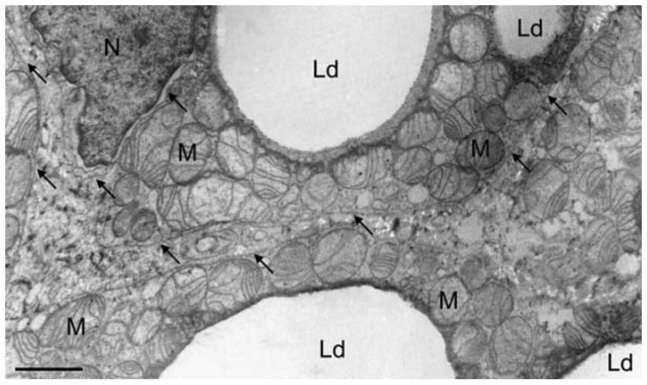
Cells in white fat tissue owe their color to the fact that they contain little else than large, single triacylglycerol droplets. Intensely colored tissues, other than the pigment cells of the skin, are rich in heme or cytochromes. This is also the case with brown fat tissue. In addition to multiple small lipid droplets (Ld), they contain an abundance of mitochondria (M), which in turn are rich in cytochromes. The mitochondria in this particular tissue also contain a high amount of an uncoupling protein (slide 6.3.1) called thermogenin.
In contrast to white fat cells, brown fat cells do not release fatty acids into the circulation but instead carry out β-oxidation themselves. The accumulated hydrogen is oxidized in the respiratory chain. The resulting proton-motive force is not used for ATP synthesis, but is instead simply dissipated as heat—the purpose of brown fat is heat production.
There is little brown fat in adult humans or most other adult mammals. However, there is a substantial amount in newborns, who because of their higher surface-to-volume ratio, and their helplessness, are at greater risk of hypothermia. Brown fat is also found in hibernating animal species such as groundhog, which need it to reheat themselves to operating temperature during arousal from hibernation.
| 10.4 |
Ketone body metabolism |
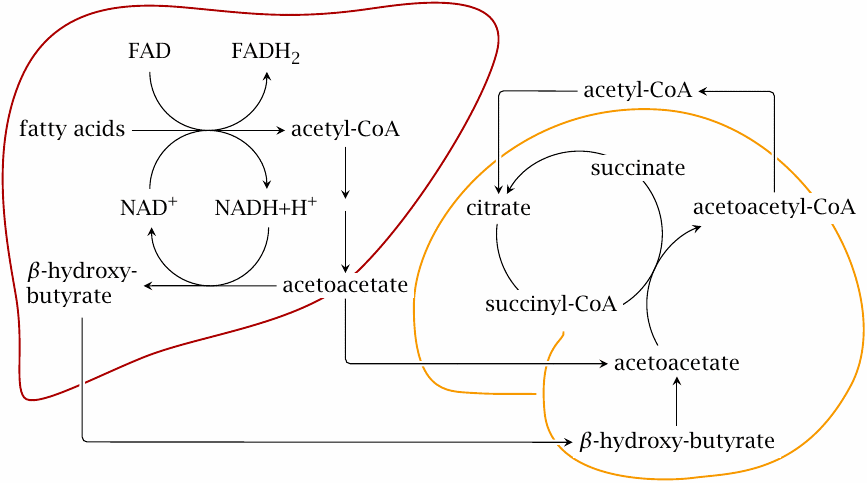
The key idea of ketone body metabolism is to convert free fatty acids into more water-soluble substrates that are easier to transport and to metabolize. This scheme gives an overview of organ relationships and pathways; note that the reactions are not stoichiometrically balanced.
Ketone bodies are formed mostly in the liver. As stated earlier, the first stage of ketogenesis consists in β-oxidation. The resulting acetyl-CoA is turned into acetoacetate along the pathway detailed in the next slide. The subsequent reduction of acetoacetate to β-hydroxybutyrate allows the liver to dispose of some of the surplus hydrogen that accumulates during β-oxidation. Both acetoacetate and β-hydroxybutyrate are released into the circulation.
Utilization of ketone bodies in the brain, muscle and other tissues is quite straightforward. β-Hydroxybutyrate is dehydrogenated again to acetoacetate, which steals coenzyme A from succinyl-CoA to become acetoacetyl-CoA. Thiolase then cleaves acetoacetyl-CoA to two molecules of acetyl-CoA, which enter the TCA cycle.
| 10.4.1 |
Synthesis of acetoacetate and β-hydroxybutyrate |
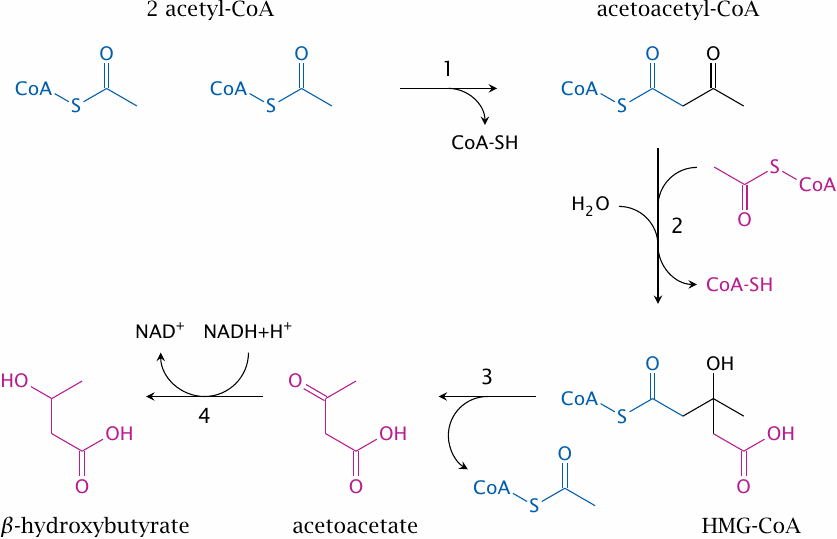
This slide details the mitochondrial pathway that converts acetyl-CoA to ketone bodies. It involves the following steps:
- 1.formation of acetoacetyl-CoA from two molecules of acetyl-CoA by thiolase. This step is the reversal of the final step in β-oxidation;
- 2.formation of hydroxymethylglutaryl-CoA (HMG-CoA) by HMG-CoA synthase;
- 3.release of acetoacetate by HMG-CoA lyase; and
- 4.reduction of acetoacetate to β-hydroxybutyrate by β-hydroxybutyrate dehydrogenase.
| 10.4.2 |
Decarboxylation of acetoacetate |
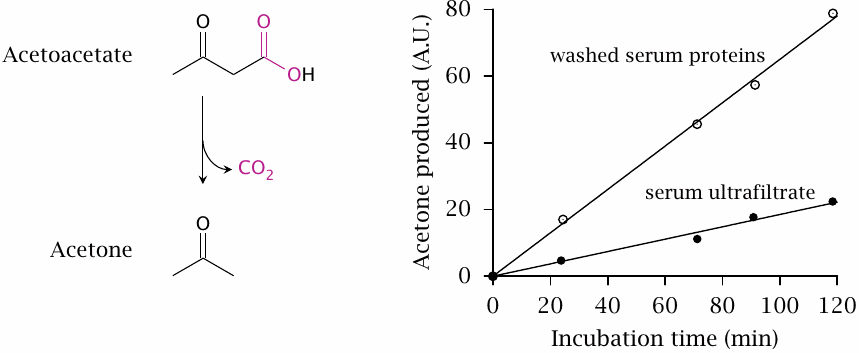
Acetone can form from acetoacetate through spontaneous, non-enzymatic decarboxylation. As shown in this plot (redrawn from [54]), the rate of decarboxylation is enhanced by unfractionated serum proteins. The catalytic activity of serum is greater when ketogenesis has been induced, suggesting the existence of a specific enzyme activity. A bacterial acetoacetate decarboxylase is known and has been studied in molecular detail; however, the hypothetical mammalian enzyme [55] has yet to be purified and characterized.
| 10.4.3 |
Acetone can serve as a precursor for gluconeogenesis |
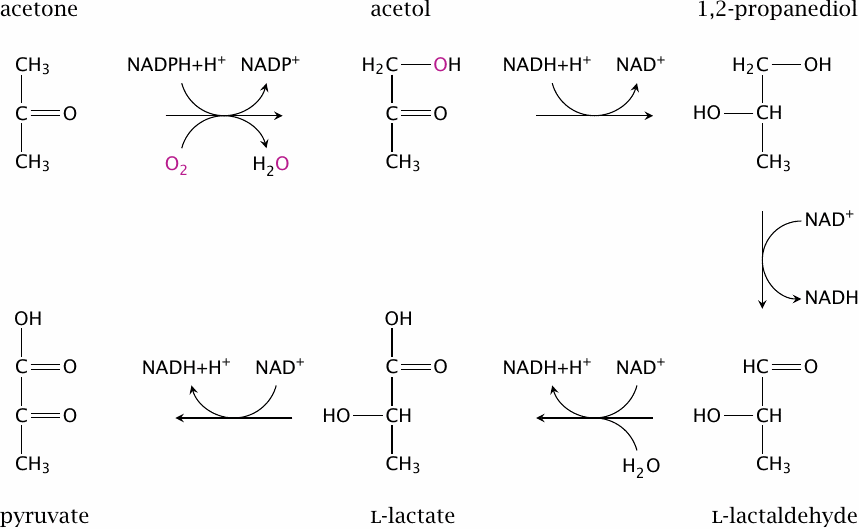
While many textbooks, even ones of recent vintage, continue to spread the myth of acetone being a useless metabolic dead end, it has been known for some time that acetone can be converted to pyruvate, which can then enter gluconeogenesis [56]. Thus, acetone provides a back door for fatty acyl carbon to be turned back into glucose. The capacity of this pathway appears to be limited; it has been estimated that up to 11% of endogenously produced glucose may be derived from acetone in fasting humans [57].
The first step of the pathway shown here is catalyzed by the enzyme cytochrome P450 type 2E1. This enzyme can also metabolize ethanol and is transcriptionally induced by both acetone and ethanol. The enzyme that converts acetol to propanediol has not been characterized; the subsequent steps are catalyzed by alcohol dehydrogenase and aldehyde dehydrogenase, respectively, which also function in the major pathway of ethanol degradation (see slide 7.4.2).64
| 10.4.4 |
Anticonvulsant effects of acetone and acetol |
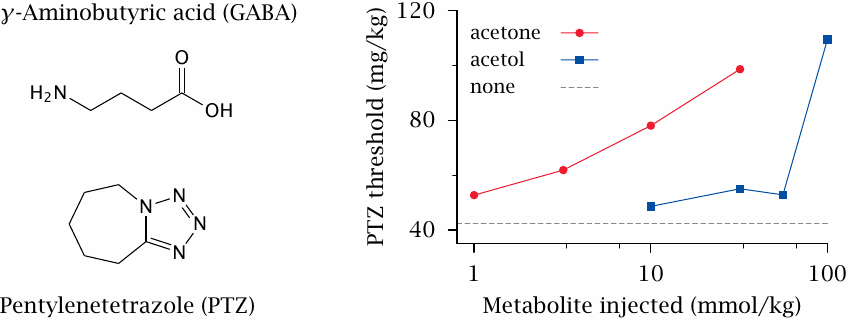
At higher than physiological plasma concentrations, acetone acts like a general anesthetic, as do many organic solvents (e.g. chloroform and diethylether). This is also the case for some antiepileptic drugs. An important common target for anesthetics and antiepileptic drugs is the GABAA receptor, which is one of the two major inhibitory neurotransmitter receptors in the central nervous system.65 Accordingly, acetone has been proposed to be responsible for the effectiveness of the ketogenic diet in epilepsy. This diet was the first effective treatment of this disease [59], and it remains in use in a significant number of patients, particularly children, who don’t respond to antiepileptic drugs.
The ketogenic diet restricts carbohydrates and protein, and it supplies most calories as triacylglycerol, much of which is then converted to ketone bodies. In animal experiments, acetone has greater antiepileptic potency than the two other ketone bodies and, as illustrated here, also than the metabolites formed in its own breakdown. In the experiment shown here [60], the GABAA receptor antagonist pentylenetetrazole was used to induce epileptic seizures. Injection of acetone raises the dosage of pentylenetetrazole necessary to trigger seizures. It does this at lower concentrations than its metabolite acetol, indicating greater anticonvulsant potency.
The ketogenic diet does not work in all patients, and it seems desirable to increase its effectiveness. A possible strategy to achieve this might be to combine the diet with inhibitors of metabolic breakdown of acetone [61]. This approach is currently undergoing experimental evaluation.
| 10.5 |
Fatty acid synthesis |
Fatty acids can be synthesized from acetyl-CoA. This is the major pathway for utilizing excess dietary carbohydrates and protein. Fatty acid synthesis occurs mainly in the fat tissue and the liver. It runs in the cytosol, which keeps it apart from mitochondrial β-oxidation. In broad outline, fatty acid synthesis is a reversal of β-oxidation: two carbon atoms at a time are added to a growing fatty acid molecule, and the new β carbon is then reduced to the alkane level. However, the mechanistic details are somewhat different.
The bulk of the work in fatty acid synthesis is accomplished by a single enzyme, fatty acid synthase, which is quite an amazing molecule: it combines six active sites with eight distinct catalytic activities on a single polypeptide chain. Its product is palmitic acid (hexadecanoic acid). As stated before, fatty acids vary in their chain lengths and degree of bond saturation. These variants are derived from palmitate through chain elongation and desaturation, which are accomplished in the ER by separate enzymes called elongases and desaturases.
| 10.5.1 |
The acetyl-CoA carboxylase reaction |
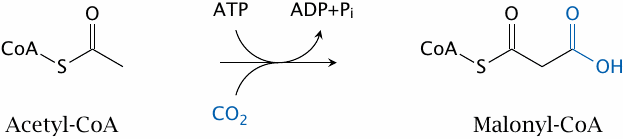
The only reaction in palmitate synthesis that is not carried out by fatty acid synthase itself is catalyzed by acetyl-CoA carboxylase. I hope that by now you recognize the pattern: CO2 is fixed, and ATP is expended—another biotin-dependent reaction, working in the same way as pyruvate carboxylase and propionyl-CoA carboxylase.
The acetyl-CoA carboxylase reaction is performed for each C2 subunit that is added to the growing fatty acid, except the very first one. As with pyruvate carboxylase in gluconeogenesis, the attachment of CO2 is transient, and one can think of it simply as an activation step that facilitates subsequent C–C bond formation.
| 10.5.2 |
The structure of fatty acid synthase |
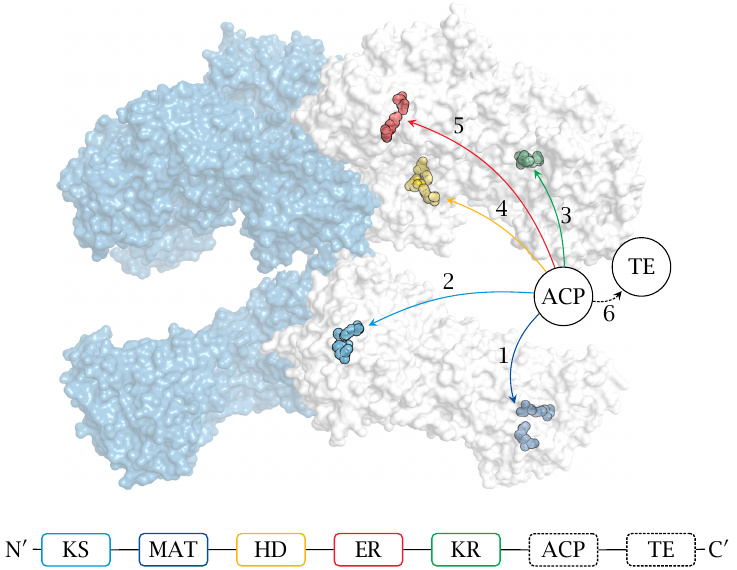
All further reactions are catalyzed by fatty acid synthase. In animals, this is a large molecule with multiple active sites located on a single polypeptide chain. Two of these polypeptides form an active dimer. The active sites are highlighted in the structure (rendered from 2vz8.pdb), and their locations along the sequence of the fatty acid synthase molecule are given as acronyms, as follows: KS, ketoacyl synthase; MAT, malonyl-acetyltransferase; KR, ketoacyl reductase; HD, hydroxyacyl dehydratase; ER, enoylreductase. The numbers indicate the place of each active site in the sequence of reactions. ACP is acyl carrier protein, and TE thioesterase; these two elements were not resolved in the crystal structure of the protein, and their locations are approximate.
| 10.5.3 |
Phosphopantetheine acts as a flexible tether in acyl carrier protein |
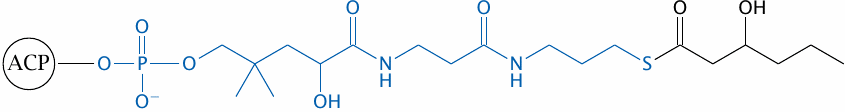
Throughout the repetitive cycle of reactions involved in the synthesis, the growing fatty acyl chain remains covalently attached to the enzyme. Attachment is made through a phosphopantetheine group, which functions as a flexible tether, allowing the acyl chain to travel and visit the various active sites on the enzyme in turn. This is reminiscent of the lipoamide moiety in pyruvate dehydrogenase and of biotin-dependent carboxylases.66 The phosphopantetheine tether group is actually not new to us, since it also occurs in coenzyme A (see slide 10.3.1).
| 10.5.4 |
Fatty acid synthase reactions (1) |
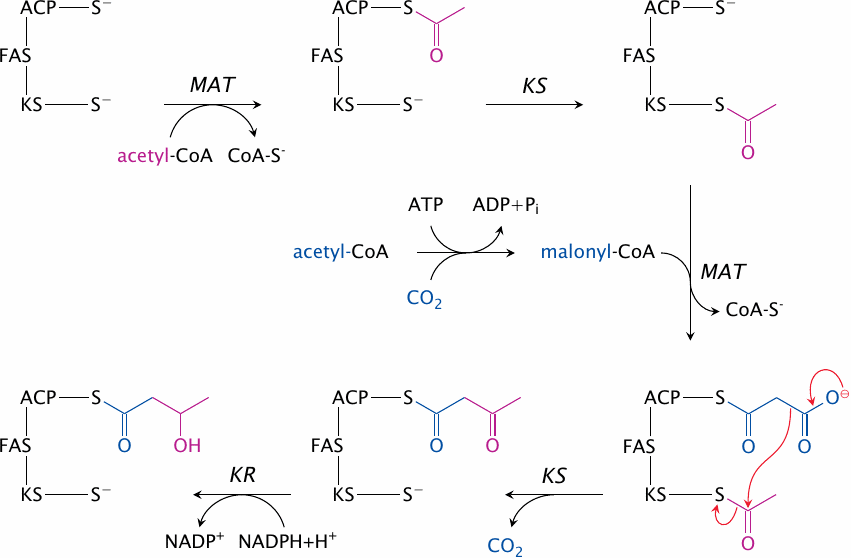
The reactions catalyzed by fatty acid synthase are shown in this slide and the next one.
- 1.Malonyl-acetyltransferase loads the first acetyl-group onto the ACP moiety. In both the substrate and the product, the acetyl group is bound as a thioester, which makes this an easy reaction.
- 2.In another thioester transfer, ketoacyl synthase acquires the acetyl group from ACP and retains it on a cysteine residue within its own active site.
- 3.Malonyl-acetyltransferase transfers a malonyl residue from malonyl-CoA (supplied by acetyl-CoA carboxylase) to ACP.
- 4.Inside the ketoacyl synthase active site, the malonyl group is decarboxylated, and the carbanion that forms in the process attacks the acetyl group that had been acquired earlier. This yields the thioester of acetoacetate.
- 5.Ketoacyl reductase converts acetoacetate to β-hydroxybutyrate.
| 10.5.5 |
Fatty acid synthase reactions (2) |
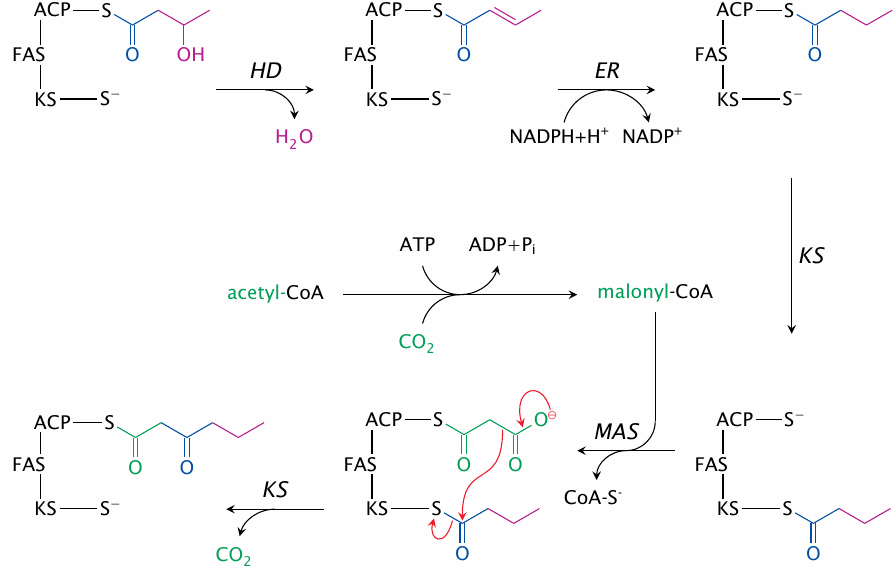
The synthesis continues from the last slide, with the following reactions:
- 6.hydroxyacyl dehydratase eliminates water, and
- 7.enoylreductase reduces the ensuing double bond. These two steps yield butyrate.
- 8.Ketoacyl synthase acquires the butyrate, freeing up ACP.
- 9.Malonyl-acetyltransferase acquires the next malonyl residue from malonyl-CoA, which is again supplied by acetyl-CoA carboxylase.
From this point on, the the cycle repeats—ketoacyl synthase decarboxylates malonate and forms the next β-keto acid, ketoacyl reductase reduces it, and so on. The cycle executes seven times overall, which results in the formation of palmitate; the palmitoyl residue is then released as free palmitate by the thioesterase activity of fatty acid synthase.
| 10.5.6 |
Mitochondrial export of acetyl-CoA via citrate |
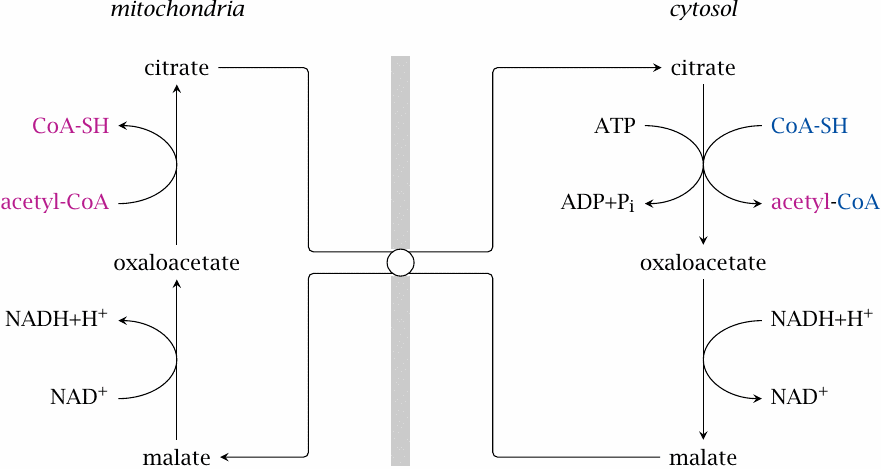
We have noted above that fatty acid synthesis occurs in the cytosol. Since acetyl-CoA is formed in the mitochondrion by pyruvate dehydrogenase, it needs to get out of the mitochondrion and into the cytosol.
We saw before that there is a similar problem in β-oxidation. In that case, the transport of acyl-CoA to the mitochondria is accomplished by the carnitine carrier system (slide 10.3.2). Since all reactions in the latter system are reversible, and acetyl-CoA is also an acyl-CoA, we might expect that the carnitine carrier system could help us out here as well. On the other hand, if transport of acyl-CoA molecules went both ways, this might facilitate a futile cycle of fatty acid synthesis and degradation.
It turns out that acetyl-CoA is indeed transported by other means. One of them is illustrated in this slide. Here, citrate is exported by the tricarboxylate carrier system. Cleavage by ATP-citrate lyase reverts the citrate synthase reaction, producing acetyl-CoA and oxaloacetate. The latter is reduced by malate dehydrogenase, and malate is exchanged for citrate. As you can see, this process will also transport one NADH equivalent to the mitochondria, which is usually accomplished by dedicated shuttle systems (section 6.9).
| 10.5.7 |
Mitochondrial export of acetyl-CoA via acetoacetate |
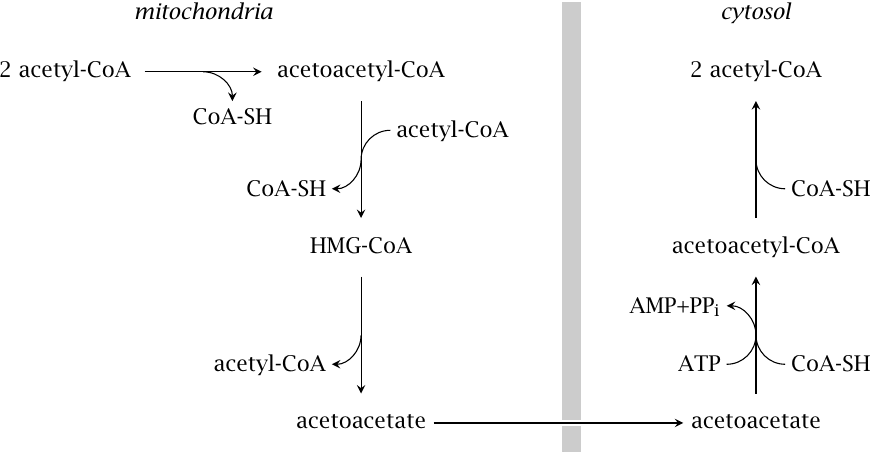
A fairly straightforward transport mechanism consists in the export of acetoacetate, which is generated in the mitochondria by the ketogenesis pathway (see slide 10.4.1). Acetoacetate can be converted back to acetyl-CoA by cytosolic acetoacetyl-CoA synthetase, which uses ATP, and then cleaved to two molecules of acetyl-CoA by thiolase. The pathway has been demonstrated in rat livers [62,63] and is also found in high activity in adipose tissue [64].
Fatty acid synthesis also requires a large amount of NADPH in the cytosol. This can be supplied by the hexose monophosphate shunt, but shuttles that export NADPH from the mitochondria also contribute (see slide 9.3.2f).
| 10.5.8 |
Elongation and desaturation of fatty acids |
- elongases reside in mitochondria and endoplasmic reticulum
- chemistry of elongation similar to β-oxidation in mitochondria, similar to fatty acid synthase in the ER
- desaturases occur in the ER, introduce double bonds at various positions
- double bonds are created at least 9 carbons away from the ω end—ω-3 fatty acids cannot be formed in human metabolism and are therefore essential
Palmitate is the end product released by fatty acid synthase; fatty acids with longer chains are formed from palmitate by separate elongase enzymes. Inside the mitochondria, the pathway of elongation is mostly a reversal of β-oxidation, except that NADPH is used instead of FADH2 in the final reduction of enoyl-CoA. In the ER, the chemistry resembles that of fatty acid synthase, except that the substrate is bound to coenzyme A rather than to the enzyme itself.
Desaturases contain iron and cooperate with several other redox-active proteins in the ER membrane; the hydrogen abstracted from the fatty acid is transferred to molecular oxygen. None of the human or animal desaturases can introduce double bonds closer than 9 carbons away from the ω end—that is, non-carboxyl end—of the substrate. Since we require ω-3 and ω-6 fatty acids as synthetic precursors of membrane lipids and of prostaglandins, such fatty acids are essential, that is, they must be obtained from the diet. All ω-3 and ω-6 fatty acids ultimately originate in plant metabolism, even if we may ingest them with animal-derived food.
| 10.5.9 |
Cerulenin, an antibiotic that irreversibly inhibits fatty acid synthase |
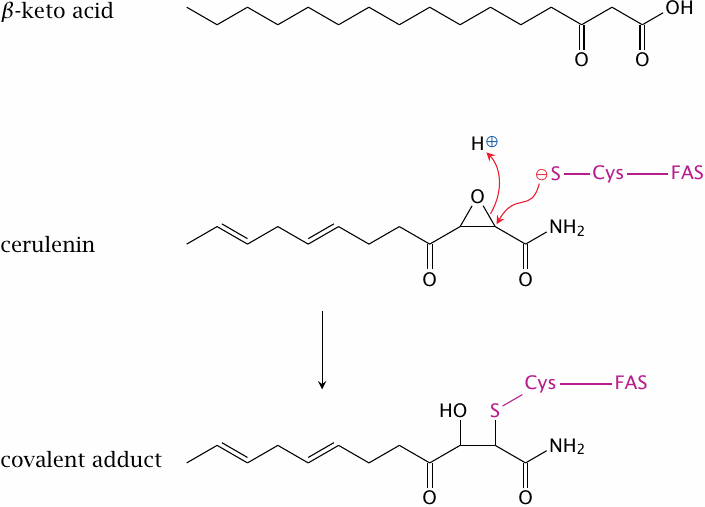
Cerulenin is a fungal antibiotic that binds and irreversibly inactivates fatty acid synthase. Its structure resembles the β-ketoacyl intermediates of fatty acid synthesis, and indeed cerulenin binds to the ketoacyl synthetase site of the enzyme. The cysteine residue in this site then reacts with the epoxide ring in cerulenin and is alkylated.
While epoxide derivatives are not terribly popular as drugs because they tend to be quite reactive and often toxic, there is presently some interest in the development of fatty acid synthase inhibitors, modeled on the structure of cerulenin, for the treatment of obesity. While this does not strike me as a particularly promising idea,67 a more interesting application is in the treatment of cancer. This is discussed in the next slide.
| 10.5.10 |
Fatty acid synthase inhibition slows tumor growth in mouse experiments |
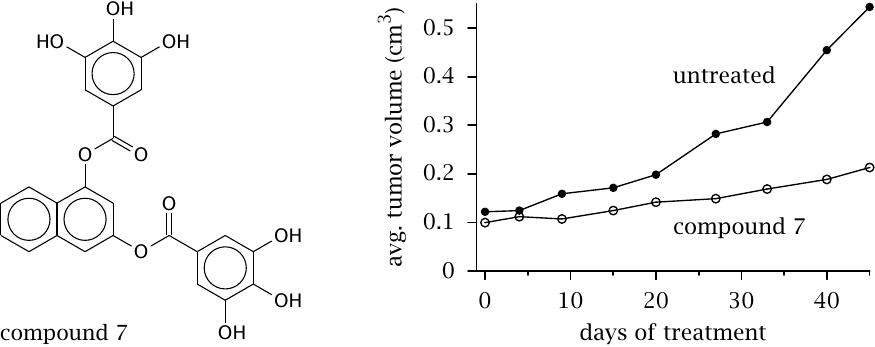
Most non-cancerous cells do not express fatty acid synthase, relying instead on fatty acids supplied by the liver and fat tissue. However, fatty acid synthase expression has been observed in cancer cells. The cells probably use these fatty acids to supply their synthesis of membrane glyco- and phospholipids, which they require for proliferation. In any event, inhibition of fatty acid synthase in tumor cells can drive them into apoptosis (programmed cell death).
This slide shows an experimental synthetic drug (with the provisional name “compound 7”) that is somewhat similar to polyphenols occurring in green tea and other natural sources. In mice experiments, it substantially reduces the growth rate of transplanted cancer. While such experimental cancers are notorious for being easier to treat than actual clinical cancer in humans, the results suggest that this line of research is worth pursuing. Figure prepared from original data in [65].
| 10.6 |
The glyoxylate cycle |
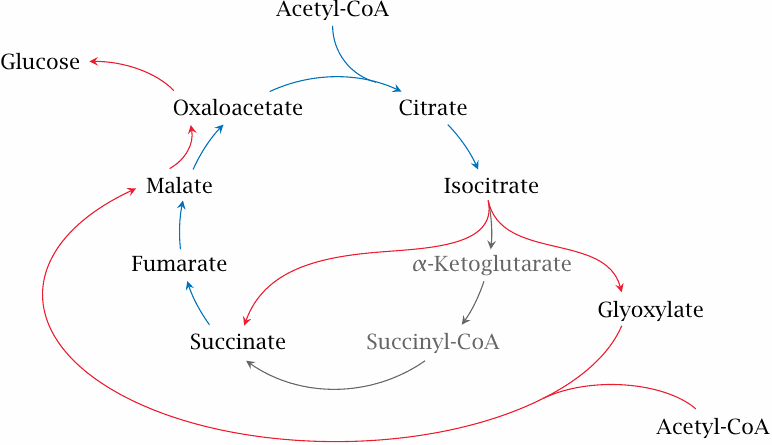
It was discussed before that carbon contained in fatty acids cannot be utilized efficiently for gluconeogenesis, since there is no efficient pathway to convert the acetyl-CoA that results from their breakdown into TCA cycle intermediates (see section 5.6 and slide 10.1.2). Interestingly, however, plants do have a straightforward pathway to do just this, namely, the glyoxylate cycle, which is an ancillary road to the TCA cycle.
In the glyoxylate cycle, the two decarboxylation steps of the TCA cycle are skipped, and an entry point for a second molecule of acetyl-CoA is created. In this manner, plants are able to use two molecules of acetyl-CoA for the net synthesis of one C4 TCA cycle intermediate.
| 10.6.1 |
Reactions in the glyoxylate cycle |
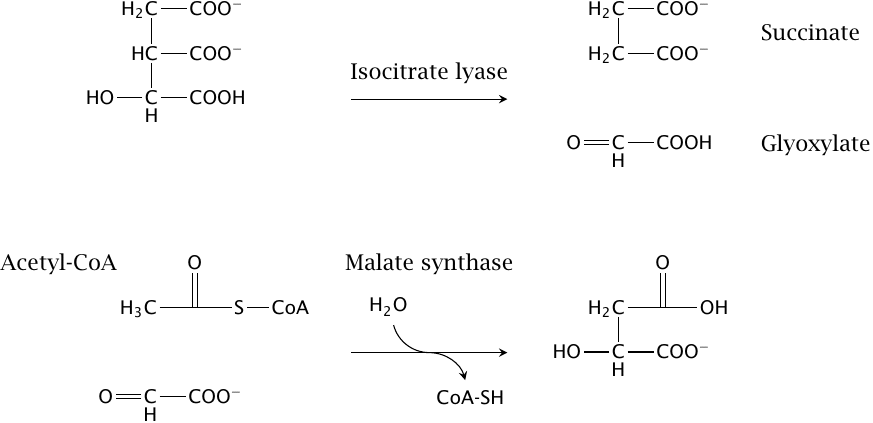
The cycle involves two reactions, both of which are mechanistically similar to citrate synthase:
- 1.Isocitrate is split into succinate and glyoxylate by isocitrate lyase. Since the isocitrate dehydrogenase and the α-ketoglutarate dehydrogenase reactions are bypassed, the loss of two carbons as CO2 is avoided; these carbons are retained in the form of glyoxylate.
- 2.Glyoxylate combines with the second acetyl-CoA molecule to form malate. This reaction is catalyzed by malate synthase, and like the citrate synthase reaction it is pushed forward by the concomitant hydrolysis of coenzyme A.
You know that many plant seeds are very rich in oil—that is, fat. The glyoxylate cycle enables plant seeds to store metabolic energy and carbon at high density as fat, and to use it for the synthesis of glucose and other carbohydrates during germination.