| 9 |
The hexose monophosphate shunt |
| 9.1 |
Outline of the pathway |
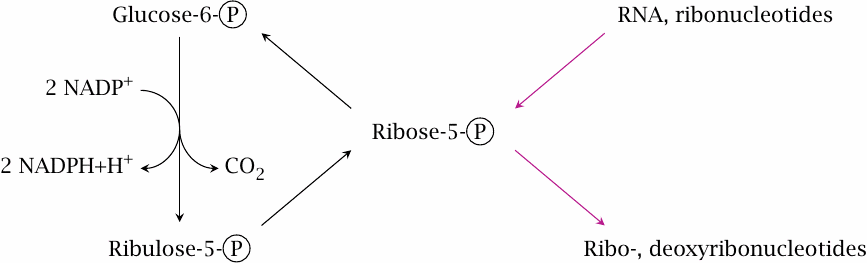
The previous chapters have shown that glucose-6-phosphate has a central place in carbohydrate metabolism. This chapter describes yet another pathway that starts with this key metabolite, namely, the hexose monophosphate shunt, or HMS for short. Since this pathway comprises both pentoses and hexoses, it is sometimes also referred to as the pentose phosphate pathway. It serves two major functions that are important for biosynthesis, namely (1)the regeneration of NADPH from NADP+, and (2)the provision or utilization of ribose. Within the pathway, we can distinguish two phases:
- the oxidative phase, in which glucose-6-phosphate is oxidized and decarboxylated to ribulose-5-phosphate, and which yields two equivalents of NADPH, and
- the regenerative “sugar shuffle” phase, which converts ribulose-5-phosphate back to 5/6 equivalents of glucose-6-phosphate.
If all the glucose-6-phosphate formed in the regenerative phase reenters the oxidative phase in each turn of the cycle, all of it will eventually be completely oxidized to CO2, with the production of 12 equivalents of NADPH. Therefore, the hexose monophosphate shunt provides an alternate pathway for the complete degradation of glucose to CO2. While the sequence of glycolysis, PDH and TCA cycle involves both the cytosol and the mitochondria, the HMS runs entirely in the cytosol. This ties in with the fact that most of the biosynthetic reactions that require NADPH also occur in the cytoplasm or in the ER, rather than in the mitochondria. It also means that the HMS is present in red blood cells, which lack mitochondria.
The oxidative and the regenerative phases of the HMS can occur at the same time, but they can also function independently from one another. This allows net flux through the pathway to follow alternate patterns in different metabolic situations:
- 1.NADPH regeneration. Complete oxidation of glucose-6-phosphate, with maximum yield of NADPH and without any net formation or utilization of other sugars, occurs when the oxidative phase and the regenerative phase occur in lockstep.
- 2.Nucleotide biosynthesis. One of the sugar phosphate intermediates of the regenerative phase is ribose-5-phosphate, which also serves as a precursor of nucleotides and nucleic acids. If required, ribose-5-phosphate can be diverted toward nucleotide biosynthesis, with a corresponding reduction in the yield of regenerated glucose-6-phosphate. Since all reactions in the regenerative phase are reversible, glucose-6-phosphate can in principle also be converted to ribose-5-phosphate without any concomitant net oxidation.
- 3.Ribose utilization. With a typical diet that is reasonably rich in starch, the net flow through the sugar shuffle will be from hexoses to pentoses. However, when eating meat only, our intake of ribose in the form of RNA will be a very significant fraction of the total dietary carbohydrates, and the net flow in the hexose monophosphate shunt will likely go the opposite way (see section 16.4).
| 9.2 |
Reactions in the hexose monophosphate shunt |
| 9.2.1 |
Reactions in the oxidative stage |
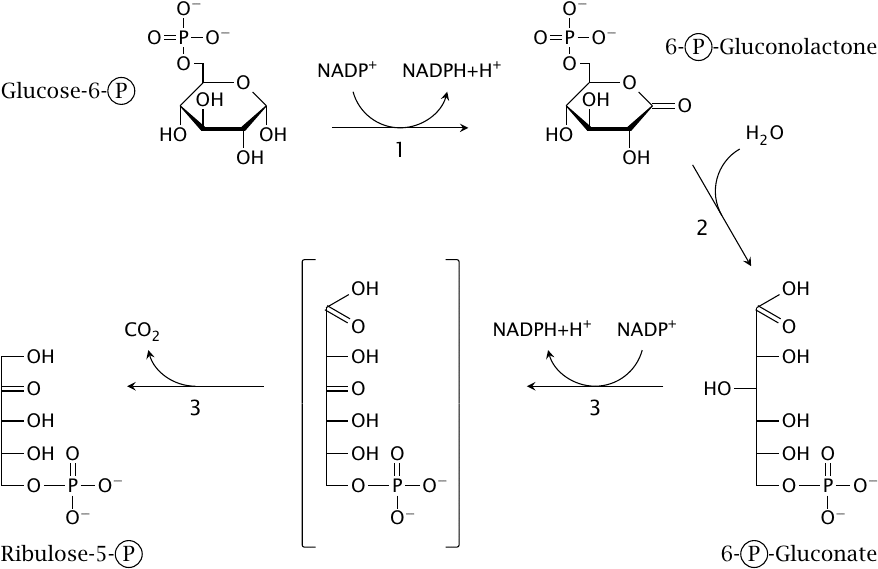
Three enzymes are required in the oxidative phase:
- 1.Glucose-6-phosphate dehydrogenase oxidizes glucose-6-phosphate to 6-phosphogluconolactone and reduces one molecule of NADP+ to NADPH.
- 2.Gluconolactonase cleaves the internal ester bond, which gives 6-phosphogluconate.
- 3.6-Phosphogluconate dehydrogenase reduces another molecule of NADP+ and decarboxylates 6-phosphogluconate to the pentose ribulose-5-phosphate.
After completion of these three initial reactions, NADPH generation is over, and all that remains is to juggle sugars in order to regenerate hexoses from pentoses.
| 9.2.2 |
Reactions in the sugar shuffle stage |
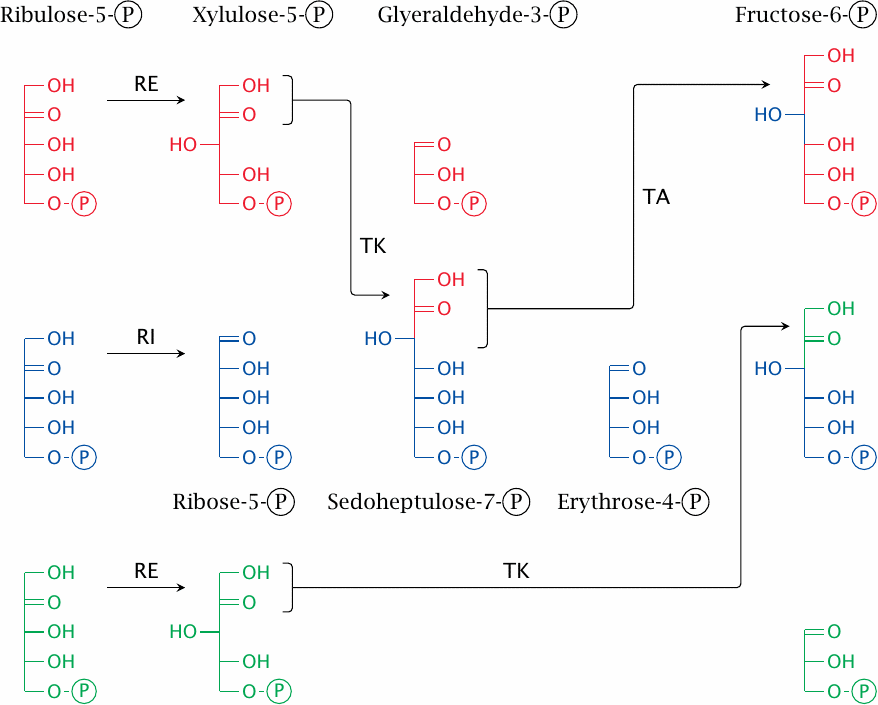
The sugar shuffle stage involves the enzymes ribulose-5-phosphate epimerase (RE), ribulose-5-phosphate isomerase (RI), transketolase (TK), and transaldolase (TA). They bring about the following reactions:
- 1.Two molecules of ribulose-5-phosphate are converted to xylulose-5-phosphate by ribulose-5-phosphate epimerase, and a third one is converted to ribose-5-phosphate by ribulose-5-phosphate isomerase.
- 2.Transketolase transfers a C2 unit from one xylulose-5-phosphate to the ribose-5-phosphate, yielding glyceraldehyde-3-phosphate and the C7 sugar sedoheptulose-7-phosphate.
- 3.Transaldolase transfers a C3 unit from sedoheptulose-7-phosphate back to glyceraldehyde-3-phosphate, which yields fructose-6-phosphate and the C4 sugar erythrose-4-phosphate.
- 4.Transketolase transfers a C2 unit from the second molecule of xylulose-5-phosphate to erythrose-4-phosphate. This yields a second molecule of fructose-6-phosphate and again glyceraldehyde-3-phosphate.
At the conclusion of the reactions depicted in this slide, three molecules of ribulose-5-phosphate have been converted to two molecules of fructose-6-phosphate and one molecule of glyceraldehyde-3-phosphate. Fructose-6-phosphate can be converted to glucose-6-phosphate in one step by phosphohexose isomerase, a glycolytic enzyme.
Conversion of glyceraldehyde-3-phosphate to glucose-6-phosphate would require several enzymes from glycolysis as well as fructose-1,6-bisphosphatase, which otherwise is required only in gluconeogenesis. I have not been able to ascertain whether or not tissues that don’t perform gluconeogenesis produce this enzyme to serve in the hexose monophosphate shunt. Also recall that two molecules of triose phosphate are needed in the aldolase reaction to form one molecule of fructose-1,6-bisphosphate. This results in an overall stoichiometry of 2.5 molecules of hexose per 3 molecules of pentose, or five hexoses per six pentoses.
| 9.2.3 |
Ketoses and aldoses in the HMS |
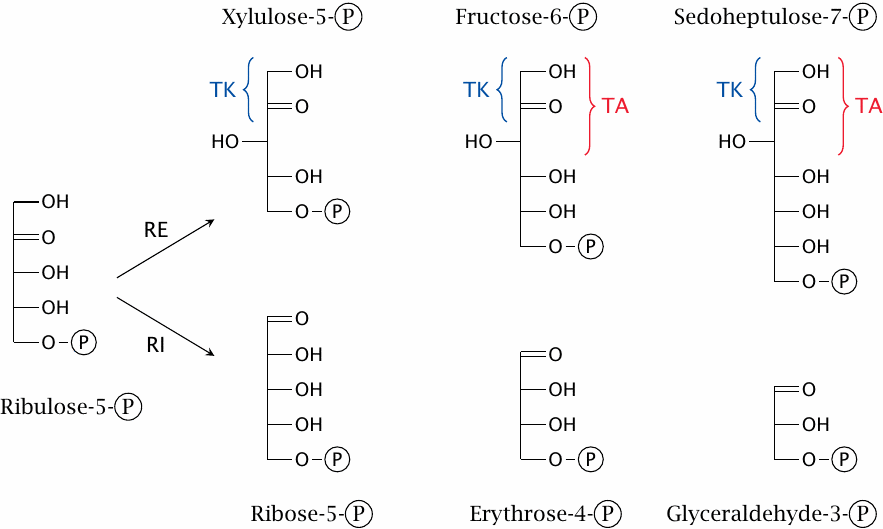
The shuffling of sugars differing in chain length is brought about by just two enzymes, namely, transaldolase and transketolase. While the sugar substrates they act upon may appear to be quite varied at first glance, they fall into just two homologous classes, within each of which the members differ only in the number of CHOH groups in their “tails”. The two enzymes only interact with the “head” parts of each of these sugar molecules, so that the chain length of the remainder doesn’t enter into the picture and does not create a need for separate enzyme specificities.
The basic idea of chain length variation is that transketolase always transfers two-carbon units, whereas transaldolase always transfers three-carbon units. A curiosity that results from this modus operandi is the occurrence of an eight-carbon sugar [45]. This molecule does not have a role in the pathway, nor does it serve any other known physiological function, and probably constitutes a byproduct that simply exists at equilibrium.
| 9.2.4 |
The mechanism of transketolase |
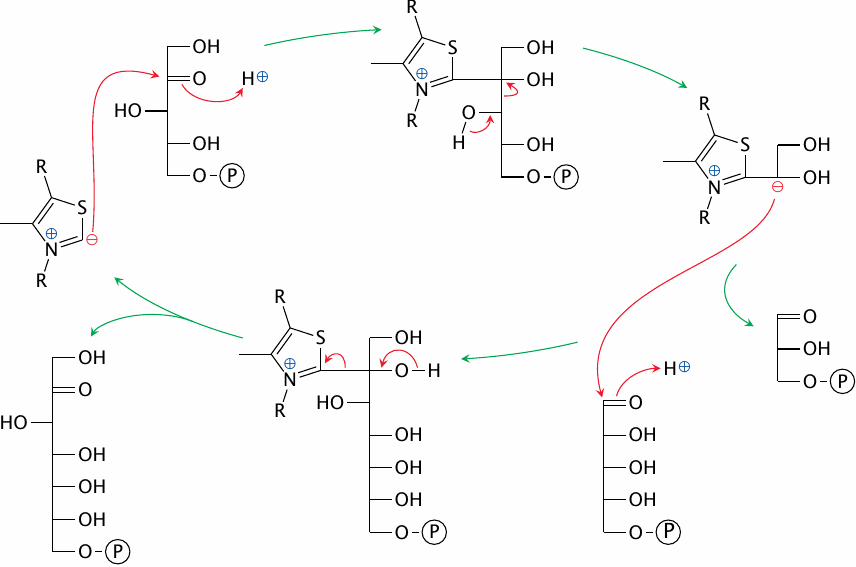
Transketolase employs the coenzyme thiamine pyrophosphate (TPP), which we encountered before in the pyruvate dehydrogenase E1 enzyme (slide 5.2.5). As in the pyruvate dehydrogenase reaction, the key function of TPP is to provide a carbanion, and as before, the carbanion reacts covalently with a carbonyl group and cleaves the adjacent C–C bond of the substrate.
It is here, however, that the similarity between transketolase and pyruvate dehydrogenase ends. In the second part of the reaction, another aldose substrate enters and carries the transiently coenzyme-bound C2 subunit away, regenerating the TPP carbanion.
| 9.2.5 |
The mechanism of transaldolase |
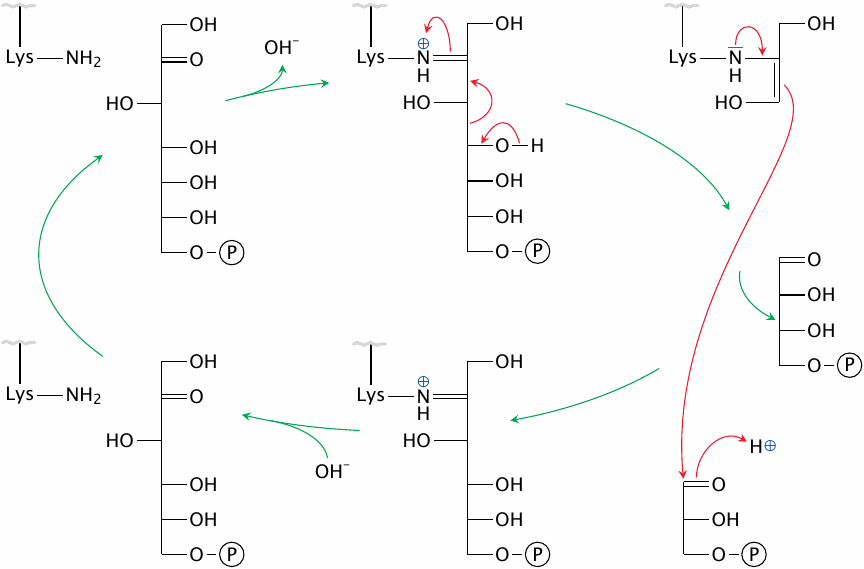
Transaldolase also forms a covalent intermediate with the fragment of the sugar molecule it transfers, and once more the carbonyl bond serves as the point of attack for cleavage. However, transaldolase cleaves the bond after the second carbon atom, resulting in the transfer of a C3 unit. The two stages of the reaction are again reversals of each other, with the exception that the two sugar substrates differ in chain length.
Considering their mechanisms, it is clear that both the transketolase and the transaldolase reactions are readily reversible. So are the isomerase reactions that interconvert the various pentose phosphates. The entire pathway therefore can proceed in either direction and bring about the interconversion of pentoses, hexoses, and sugars of other lengths, in any amounts and proportions as needed.
| 9.3 |
The physiological role of NADPH |
Our discussion of the HMS pathway is now complete, and we will now have a look at the various metabolic functions of NADPH.
| 9.3.1 |
Why do we need both NADH and NADPH? |
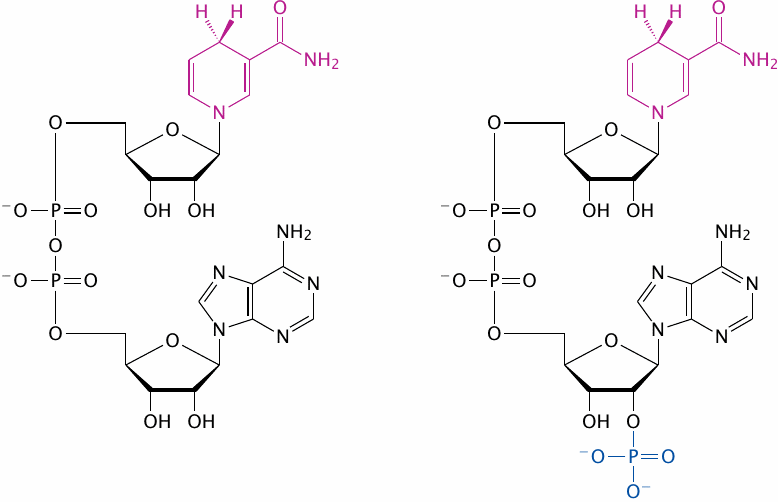
Why is NADPH needed in addition to NADH? The two coenzymes only differ by one phosphate group, and that group is far away from where the action is: The redox-active group is the pyridine ring in the nicotinamide moiety (highlighted), whereas the extra phosphate in NADP is located on the adenosine moiety at the other end of the molecule.
While the phosphate group does not make any difference to the redox chemistry performed by the two coenzymes,53 it enables them to interact with separate sets of enzymes. Consider that all enzymes which consume or regenerate NAD+ will share the same pool of the cosubstrate, and the reaction equilibria of all of them will be affected by the same ratio of oxidized to reduced form, [NAD+]/[NADH].
The extra phosphate group on NADP allows it to interact with another, different set of enzymes. Therefore, because the coenzymes participate in separate sets of equilibria, they can themselves be maintained in different redox states. To use a simile: the two coenzymes are like two different currencies—both are money, but it is possible to tune the cost of borrowing of each separately to different economic objectives. Inside the cell, NAD is mostly oxidized. The ready availability of NAD+ will help to speed up the oxidative reactions in the TCA cycle and glycolysis. In contrast, NADP is mainly found in the reduced state, which will promote reductive reactions in biosynthesis.
The choice of of either NAD or NADP as the cosubstrate will not only affect the turnover rate of a redox reaction but also its free energy (ΔG). According to [46], the [NADH]/[NAD+] ratio in the cytosol is 0.001, while the [NADPH]/[NADP+] ratio is 100. Neglecting the very slight difference in ΔG0, the 105 times higher relative abundance of NADPH works out to a difference of ~30 kJ/mol in actual ΔG. This amount of energy is similar to that released by the hydrolysis of ATP to ADP, which is not a coincidence (see next slide).
| 9.3.2 |
NADPH generation by malic enzyme |
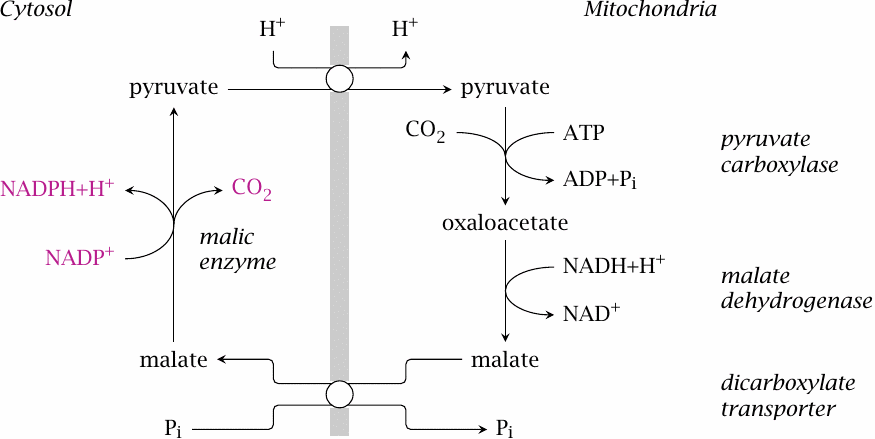
While the HMS is the major source of NADPH in most tissues, some other pathways contribute to the supply, particularly in tissues that synthesize fatty acids or sterols.
The shuttle shown here reuses most elements of the one that moves oxaloacetate from the mitochondria to the cytosol for gluconeogenesis (see slide 7.4.1, which also shows how to balance the transport of phosphate). However, in the final step of the shuttle considered here, cytosolic malate is not converted to oxaloacetate but is instead decarboxylated by malic enzyme, which generates NADPH; the pyruvate that is left over can reenter the mitochondria.
On the mitochondrial side, the shuttle consumes one equivalent of NADH and ATP each. The ATP drives the carboxylation of pyruvate, which is reverted by malic enzyme. Assuming that malic enzyme can recover the full free energy acquired from ATP during carboxylation and apply it toward the reduction of NADP+, the enzyme should indeed be able to raise the ratio of cytosolic NADPH to NADP+ to beyond that of NAD by the factor of 105 explicated in section 9.3.1. While this assumption may be a bit optimistic, it is also not strictly necessary, since the [NADH]/[NAD+] ratio is significantly higher in the mitochondria than in the cytosol, which helps push the cycle in the indicated direction. Overall, the participation of ATP in this cycle accounts for the difference in concentration and free energy between NADPH and NADH discussed above.
| 9.3.3 |
NADPH generation by transhydrogenase and NADP-linked isocitrate dehydrogenase |
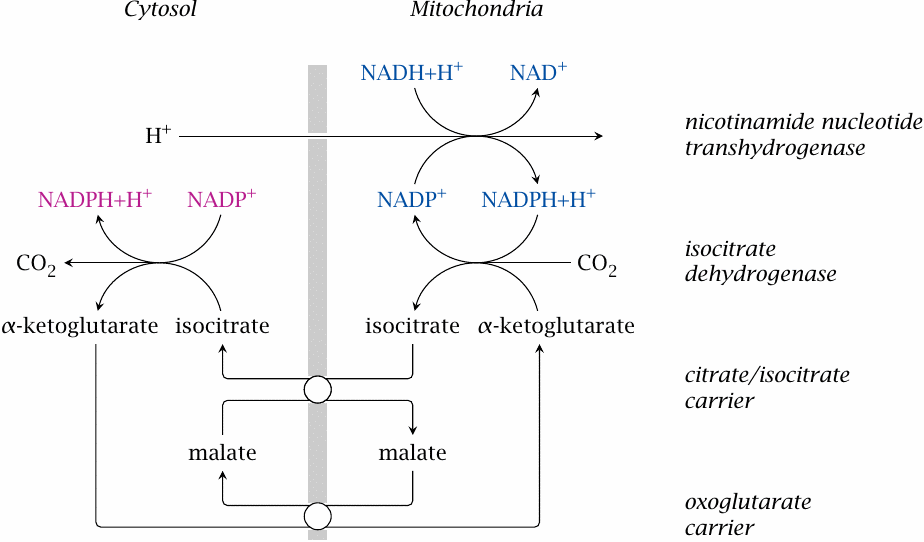
We have already encountered nicotinamide nucleotide transaminase as a source of mitochondrial NADPH before (see slide 6.10.2). From the mitochondria, NADPH can be transported to the cytosol by the concerted action of NADP-dependent isocitrate dehydrogenases on both sides of the membrane and of two mitochondrial carriers, which together manage to exchange isocitrate for α-ketoglutarate across the membrane.
Note that this cycle does not involve the hydrolysis of ATP. The single proton imported by the transhydrogenase does not provide quite the same amount of free energy as ATP, and the question therefore arises how sufficient driving force for the overall process is derived. As far as I can see, the only other contributing factor is the more reduced state of NAD inside the mitochondrion as compared to the cytosol.54 It may thus be that this shuttle works only when both the proton-motive force and the mitochondrial NADH are at high levels, while cytosolic NADPH is lowered due to high rates of consumption, such as for example during fatty acid synthesis in fat tissue [47].
| 9.3.4 |
Uses of NADPH |
- synthesis of fatty acids and cholesterol
- fixation of ammonia by glutamate dehydrogenase
- oxidative metabolism of drugs and poisons by cytochrome P450 enzymes
- generation of nitric oxide and of reactive oxygen species by phagocytes
- scavenging of reactive oxygen species that form as byproducts of oxygen transport and of the respiratory chain
The first three topics on this list will be covered in the later chapters on the metabolism of triacylglycerol, cholesterol, amino acids, and drugs, respectively. Here, we will briefly look at the roles of NADPH in the formation of nitric oxide and in the formation and the scavenging of reactive oxygen species.
| 9.3.5 |
The nitric oxide synthase reaction |
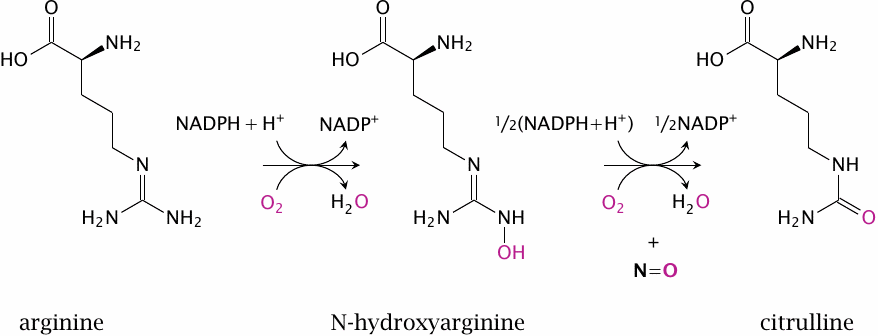
Nitric oxide is synthesized intracellularly by nitric oxide synthase (NOS). This reaction is rather complex and involves two successive monooxygenase steps. In the first step, arginine is converted to N-hydroxyarginine (NOHA), which is cleaved in the second step to NO and citrulline.
NOS occurs in several variations. Endothelial NOS (eNOS) and neuronal NOS (nNOS) are found in the cell types indicated by their names. Inducible NOS (iNOS) is found mainly in inflammatory cells. All these enzymes are homologous and perform the same reaction, but they differ in their regulatory properties.
| 9.3.6 |
Signaling effects of nitric oxide |
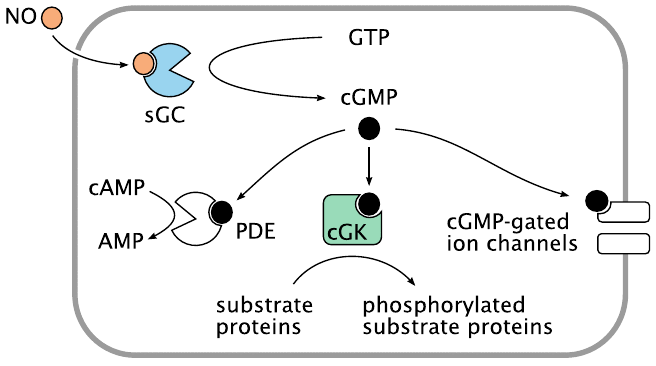
Nitric oxide produced by NOS diffuses out of the cell of origin, for example a vascular endothelial cell, and then into another one, such as a vascular smooth muscle cell. Within its target cell, NO binds and activates soluble guanylate cyclase (sGC), which then begins to make cyclic GMP (cGMP). Like cAMP, cGMP acts as a second messenger inside the cell.
Also like cAMP, cGMP targets multiple effector molecules. The activation of cGMP-dependent protein kinase (cGK) results in the phosphorylation of various proteins. In vascular smooth muscle, this induces relaxation, which in turn lowers the blood pressure; this is exploited by NO-releasing drugs in the treatment of hypertension. Phosphodiesterase 5 (PDE) is activated by cGMP, too, and begins to degrade both cAMP and cGMP. Actuation of cyclic nucleotide-gated cation channels affects the membrane potential and the cellular calcium level.
| 9.3.7 |
Phagocytes use NADPH to generate reactive oxygen species |
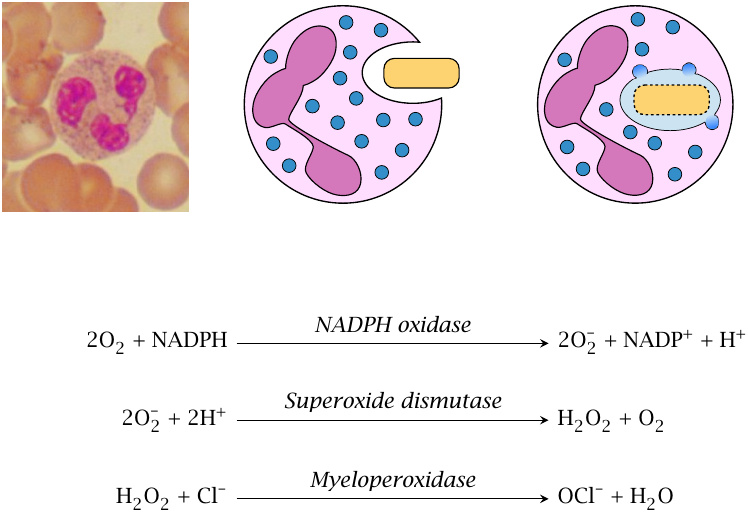
Neutrophil granulocytes (depicted) and macrophages ingest bacteria and then fuse the endocytotic vacuole with granules that contain various kinds of antimicrobial molecules. Among these, there are several enzymes that produce reactive oxygen species. The first such enzyme is NADPH oxidase, which converts molecular oxygen to superoxide. Subsequently, superoxide dismutase and myeloperoxidase produce H2O2 and HOCl. All of these reactive oxygen species (ROS) have powerful antimicrobial activity, and patients with defects in NADPH oxidase or myeloperoxidase are prone to severe bacterial infections.
Superoxide can also combine with nitric oxide to form peroxynitrite, another molecule with strong antimicrobial activity. This is one function of the NO generated in macrophages by inducible nitric oxide synthase (iNOS).
The reactions involved in ROS generation by phagocytes are discussed in some more detail in section 18.3.
| 9.3.8 |
Scavenging of reactive oxygen species requires NADPH, too |
Where not needed for immune defense, reactive oxygen species are harmful rather than useful; for example, they can react with non-saturated fatty acyl residues in lipid membranes (see below). Nevertheless, some ROS continually form as byproducts of respiration and also of oxygen transport in erythrocytes, since binding to hemoglobin offers O2 an opportunity to steal an electron from the heme and turn itself into superoxide.
ROS toxicity is kept in check by glutathione (G-SH), which is present in the cells at low millimolar concentrations. An important step in the detoxification of ROS is the reduction of hydrogen peroxide to water, which is catalyzed by glutathione peroxidase or peroxiredoxin. In the process, glutathione or peroxiredoxin are oxidized to their disulfide forms. These are reduced again by glutathione reductase and thioredoxin, respectively, both of which require NADPH (see section 18.7).
| 9.4 |
Glucose-6-phosphate dehydrogenase deficiency |
- most patients are healthy most of the time—hemolytic crises occur upon exposure to drugs or diet components that cause enhanced formation of ROS
- manifest in red blood cells because these cells lack protein synthesis—no replacement of deficient protein molecules
- affords partial protection against malaria—similar to sickle cell anemia and other hemoglobinopathias
- X-chromosomally encoded—males more severely affected
Red blood cells lack mitochondria and thus depend entirely on the HMS for NADPH regeneration. Mutations in glucose-6-phosphate dehydrogenase—the first enzyme in the pathway, see section 9.2—that reduce its activity limit the supply of NADPH and, therefore, the capacity of the cell to detoxify reactive oxygen species. ROS that go unscavenged will cause the peroxidation of membrane lipids (see section 18.5 for the mechanism) and destroy the cell. This condition—namely, the occurrence of episodes of hemolytic anemia upon ingestion of ROS-inducing foods or drugs—is called favism.
Glucose-6-phosphate dehydrogenase is encoded on the X chromosome, and accordingly males (who have only one X chromosome) are affected the most. However, heterozygous females are not exempt, since, in keeping with the “Mary Lyon hypothesis,” one of the two X chromosomes in females is inactivated randomly early during embryonic development [48]; therefore, in heterozygous females, the bone marrow precursors of the red blood cells will represent a mosaic of intact and deficient genes. Indeed, the observation of such a mosaic in glucose-6-phosphate dehydrogenase deficiency was the first proof of random X chromosome inactivation in humans [49].
| 9.4.1 |
Vicia faba and favism |
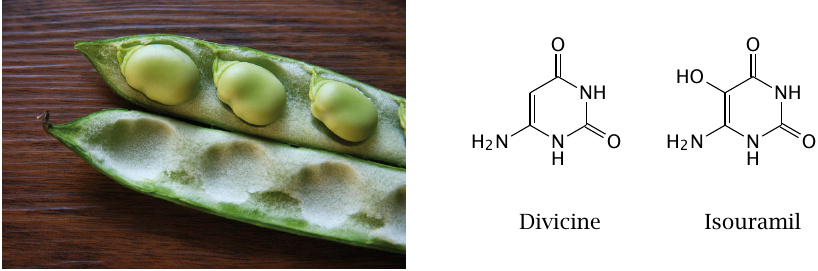
The word “favism” derives from the Latin name of the broad bean, Vicia faba. Broad beans contain several pyrimidine derivatives such as isouramil and divicine that can catalyze the formation of ROS through redox cycling, which is explained in the next slide.
| 9.4.2 |
Redox cycling of isouramil |
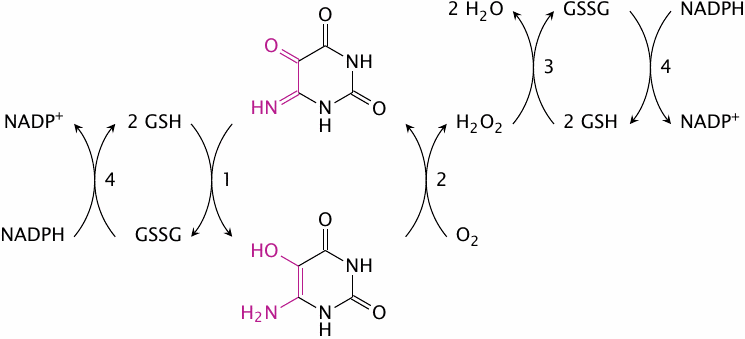
This scheme outlines the redox cycle induced by isouramil. Reactions (1) and (2) occur spontaneously, without any need for enzymatic catalysis. The H2O2 formed in reaction (2) is reduced by glutathione peroxidase (3). The glutathione disulfide formed in reactions (1) and (3) is reduced at the expense of NADPH in reaction (4) by glutathione reductase. Therefore, this redox cycle consumes four equivalents of reduced glutathione (GSH) in every full turn [50]. Divicine and some other substances contained in broad beans cause analogous cycles.
| 9.4.3 |
Malaria parasites detoxify heme by crystallization |
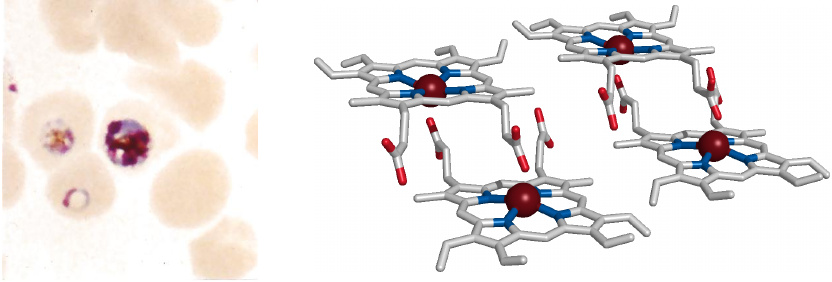
Malaria parasites multiply inside erythrocytes, where they feed on hemoglobin. They digest the protein (globin) but leave behind the heme, which is toxic to them because it catalyzes the formation of reactive oxygen species. To reduce the toxicity of heme, the parasites induce its crystallization; the crystalline deposits are visible inside the infected cells as brownish so-called malaria pigment or hemozoin. The parasites also produce several heme-binding proteins to aid in the detoxification.55
Another factor that may contribute to the protection of the parasites from heme toxicity is the red cells’ own glutathione-dependent ROS scavenging pathway. This provides a plausible explanation for the observation that glucose-6-phosphate dehydrogenase deficiency affords partial protection from malaria, which has led to the enrichment of this enzyme defect in the human gene pool in endemic malaria areas.
| 9.4.4 |
Primaquine and glucose-6-phosphate dehydrogenase deficiency |
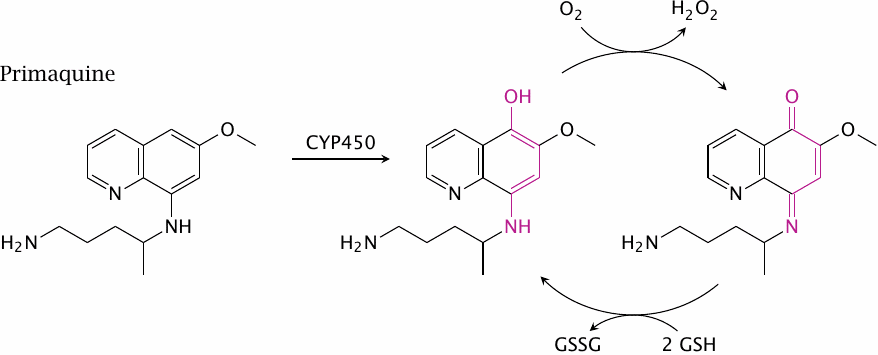
The higher prevalence of glucose-6-phosphate dehydrogenase deficiency in people from traditionally malaria-infested areas is something to keep in mind when treating malaria patients with primaquine. The drug is hydroxylated to 5-hydroxyprimaquine by cytochrome P450 enzymes, and the metabolite will set up a redox cycle that is analogous to the isouramil-based one shown in slide 9.4.2 (but is only shown partially in this slide). Primaquine thus may induce hemolytic crises in these patients. Several other drugs can do the same.