| 1 |
Introduction |
| 1.1 |
Motivation: Why would you study metabolism? |
Long answer: You have a generous and warm character, and you have spent too much time in front of the family TV set. You are therefore determined to become a famous doctor and save many, many lives every hour of the day, without asking anything in compensation but the admiring gazes of the populace, and may be a Rolls Royce. Metabolism is an ever so tiny part of the vast knowledge you have set out to master in order to fulfil your destiny.
Wrong? Alternate long answer: You have the inquisitive mind of a Sherlock Holmes and the financial savvy of a Howard Hughes, and you have determined that soaking medical doctors for damages is the best road to wealth and fame. Understanding the biochemical basis of medicine will help you to stun your audiences in court and grind the defendants and their counsels into the dust.
Wrong again? Then try the short answer: You want to pass your exam.
| 1.2 |
Significance of metabolism in medicine |
- hereditary enzyme defects
- diabetes, atherosclerosis, gout
- antimetabolites in the chemotherapy of cancers and infections
- inactivation and elimination of xenobiotics and drugs
Metabolism is a central theme in biochemistry; it keeps cells and organisms alive, by giving them the energy they need to carry on and the building blocks they require for growth and propagation. Metabolism is also an important theme in medicine and pharmacy. Genetic defects of metabolic enzymes, while not among the most common forms of disease, are nevertheless common enough to warrant the routine screening of newborns. Knowledge of the metabolic pathways that will be affected by a genetic enzyme defect is important to understand its clinical manifestations and to devise strategies for proper diagnosis and treatment.
While metabolic diseases that result from single enzyme defects are comparatively rare, there are metabolic diseases which are much more common, such as diabetes mellitus and gout. Atherosclerosis, which is even more common, is not a purely metabolic disease; however, its initiation and progression are determined to a large extent by metabolic factors. In all these cases, an understanding of the underlying metabolic aspects is the basis for prevention and treatment.
Even with diseases that are not primarily due to metabolic aberrations, metabolic pathways often provide important targets for drug therapy. Cases in point are malignant tumors and autoimmune diseases, which are commonly treated with antimetabolites that disrupt cell proliferation and promote programmed cell death (apoptosis). Antimicrobial drugs often target enzymes in vital metabolic pathways of bacteria and parasites. Conversely, many drugs that target receptors other than enzymes require metabolic modification for activation or elimination.
From all this, it should be quite clear that metabolism matters to all those who pursue a career in medicine or a related field, and who want to truly understand what they are doing. These lecture notes aim to supply this required foundation.
| 1.3 |
Catabolic and anabolic reactions |
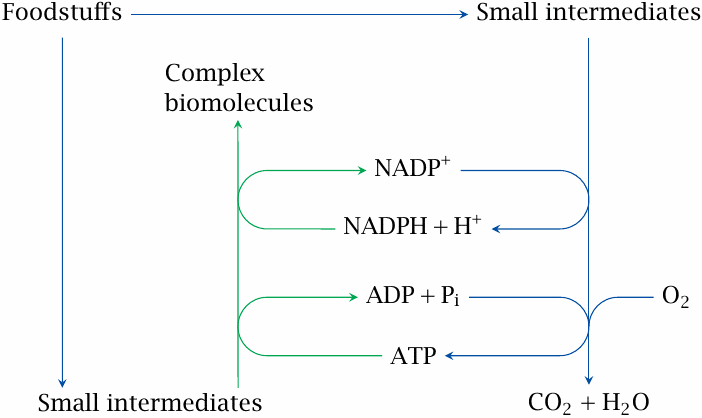
The metabolism of animals and humans can be divided into catabolic reactions (blue arrows) and anabolic ones (green). The word “catabolic” means the same as “degradative,” but it is Greek and therefore sounds a whole lot more erudite and scholarly. A large share of the substrates broken down in catabolism are used for producing ATP, the “electric energy” of the cell. Just as electricity can be used to drive just about any household job, ATP is used for almost every energy-requiring task in cell biology. Because of its key role in the life of the cell, we will devote a good deal of space—chapters 3–6—to the metabolic pathways that allow the cell to regenerate ATP.
The word “anabolic” might be translated as “constructive.” Anabolic pathways are the opposite of catabolic ones, that is, they create new biomolecules. They produce small molecules and building blocks that are not sufficiently available in the food, as well as macromolecules, in particular proteins and nucleic acids. Apart from building blocks and ATP, anabolic pathways also require a good deal of reducing power, mostly in the form of NADPH. One major pathway that supplies NADPH is the hexose monophosphate shunt, which is covered in chapter 9.
Some pathways can function both in a catabolic and an anabolic context. An example is the citric acid cycle, which breaks down acetyl-CoA but also participates in the synthesis of amino acids. Such pathways are sometimes referred to as amphibolic.
| 1.4 |
Diversity of metabolism: pathways in plants and bacteria |
| Pathway | Organisms |
| photosynthesis | plants and cyanobacteria |
| nitrogen fixation | specialized soil bacteria |
| oxidation or reduction of inorganic minerals | archaebacteria |
| acid- and gas-producing fermentations | anaerobic bacteria |
While the scope of this text is mostly restricted to human metabolism, it is useful to take a brief look beyond these confines. There are several mainstream metabolic pathways that occur in all classes of living organisms. A good example is glycolysis, the main pathway of glucose degradation, which is found all the way up from Escherichia coli to Homo sapiens. On the other hand, some of the metabolic processes in plants or in distinct groups of microbes are quite different from those found in man or animals.
Photosynthesis enables plants to create glucose—and from it, the carbon skeletons of all their other metabolites—from nothing but CO2 and water. The same is true of blue-green algae or cyanobacteria.1 Organisms incapable of photosynthesis are heterotrophic, which means that they must feed on other organisms. In contrast, photosynthesis makes organisms autotrophic, that is, capable of feeding themselves.2 Note, however, that plant life also depends on pathways other than photosynthesis. An example is the degradation of starch, which is stored in large amounts in plant seeds such as wheat and rice as well as in bulbs such as potatoes. The pathways of starch utilization employed by plants are analogous to those found in animals.
Nitrogen fixation, that is, the reduction of atmospheric nitrogen (N2) to ammonia (NH3), is performed by the bacterium Sinorhizobium meliloti and related soil bacteria. All other living organisms require nitrogen in already reduced form, and therefore depend on these bacteria. The word Rhizobium—the former first name of this bacterium—means “living on roots”. Sinorhizobium meliloti thrives on the roots of plants, which take up the surplus ammonia supplied by the bacteria and utilize it for synthesizing their own amino acids; the plants, in turn, provide the bacteria with a nutrient-rich environment. This symbiotic process is common with legumes such as alfalfa, soy beans, and peas. Including these plants in crop rotation schemes helps to keep the soil supplied with reduced nitrogen. Alternatively, the nitrogen fixation bottleneck can be bypassed altogether by supplying reduced nitrogen with chemical fertilizers.3
Some archaebacteria, which live in exotic environments such as submarine volcanic hot springs, have developed correspondingly exotic metabolic pathways. For example, some of these organisms are capable of extracting energy from the oxidation of iron or the reduction of sulfur.
While all these pathways are certainly very interesting, we will not consider them any further in these notes. Instead, we will confine the discussion to the major metabolic pathways that occur in the human body. We will also relate these pathways to human health and disease, and to some of the therapeutic strategies that have been developed for metabolic diseases.
In the remainder of this chapter, we will start with a broad overview of foodstuffs and their digestion and uptake in the intestinal organs. This will provide some important context for the detailed discussion of metabolic pathways in the subsequent chapters.
| 1.5 |
Types of foodstuffs |
- carbohydrates
- protein
- fat
- nucleic acids
The three major categories of foodstuffs relevant to human metabolism are named on every box of cereal or cup of yogurt; and in case you do not remember them, I suggest you run out right now to buy some such educational piece of grocery; dollar for dollar, its educational value might exceed that of your attendance of this class.
The fourth item in the list above, nucleic acids, could as well have been subsumed under carbohydrates, since only the ribose and deoxyribose contained in them have significant nutrient value. The pathways that would allow the reuse of the bases for nucleotide and nucleic acid synthesis exist in principle, but experimental studies indicate that ingested bases are mostly degraded and excreted (see section 16.4).
| 1.5.1 |
Breakdown of foodstuffs: Overview |
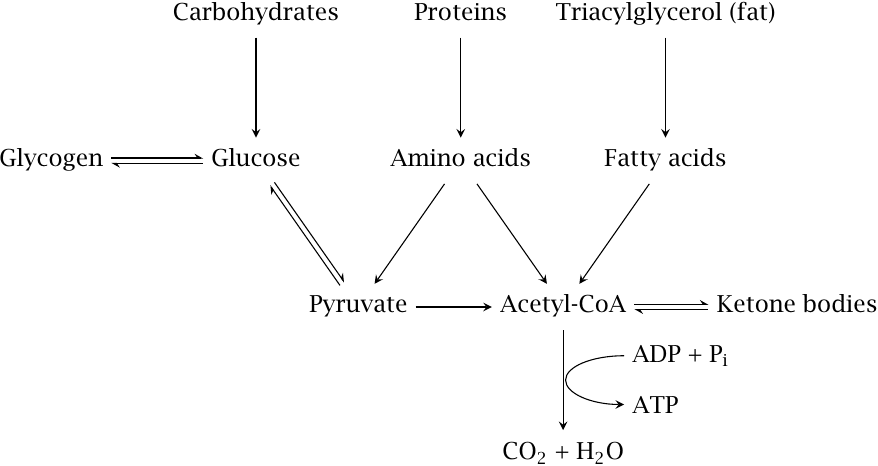
In the first stage of their utilization, all foodstuffs are split into their building blocks; this happens mostly during digestion in the small intestine. After the building blocks—mainly glucose, amino acids, and fatty acids—have been taken up and distributed through the blood stream, complete breakdown to CO2 and H2O proceeds intracellularly via pyruvate and acetyl-CoA, which function as central hubs of foodstuff utilization.
Glucose can transiently be stored in polymeric form as glycogen, which evens out the peaks and valleys of glucose supply during the day. If required, additional glucose can be produced via gluconeogenesis from amino acids whose degradation yields pyruvate.
When other forms of substrate carbon are in short supply, fatty acids—either taken up with the food or released from fat tissue—can be converted via acetyl-CoA to ketone bodies, which represent a more water-soluble transport form of carbon than the fatty acids themselves.
This slide is of course simplified and contains several approximations. For example, some carbohydrates do not directly yield glucose upon depolymerization; these may then be converted to glucose through dedicated adapter pathways. Similarly, the breakdown of some amino acids does not yield pyruvate or acetyl-CoA but instead produces citric cycle intermediates. Like pyruvate, these intermediates can also be converted to glucose if needed.
| 1.6 |
Functional anatomy of the digestive system |
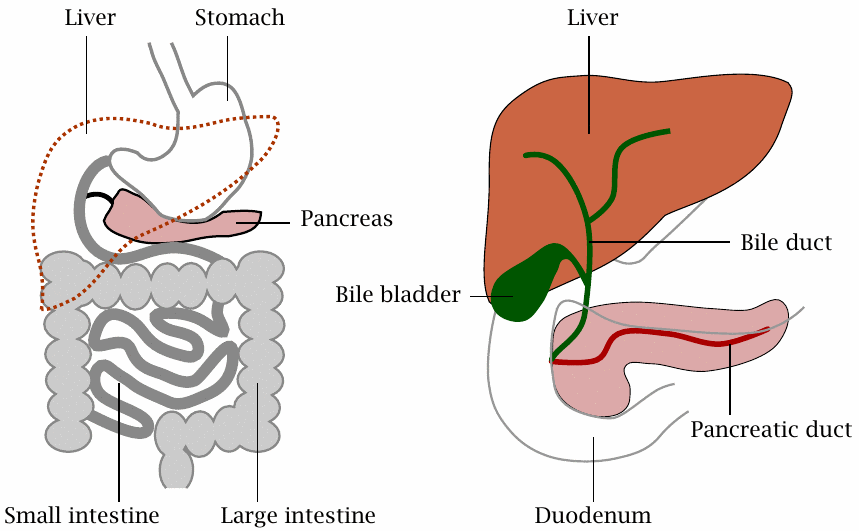
The digestive system contains the intestinal hollow organs, that is, the esophagus, stomach, small and large intestine. In addition, it also comprises the pancreas and the liver, both of which arise through budding and outgrowth from the primordial intestine during embryonic development.
| 1.6.1 |
Intestinal organs: functional overview |
| Organ | Function |
| stomach | killing of microbes contained in the food; protein denaturation |
| small intestine | breakdown of macromolecules to small molecules, uptake of the latter |
| large intestine | fluid and ion reuptake |
| pancreas | production of digestive enzymes and of hormones |
| liver | production of bile; metabolic homeostasis |
The mucous membrane of the stomach produces gastric acid, HCl, which denatures proteins and kills microbes contained in the food. The pancreas supplies most of the digestive enzymes, whereas the liver provides bile acids, which are essential for the solubilization of fat. The bile and the pancreatic juice also contain large amounts of sodium bicarbonate, which neutralizes the gastric acid; the milieu inside the small intestine is slightly alkaline.4
The digestive enzymes secreted by the pancreas into the gut do most of the work involved in digestion of ingested foodstuffs. Therefore, depolymerization of foodstuff macromolecules occurs extracellularly.5
An obvious answer is that there are no transport mechanisms for the uptake of macromolecules across the cell wall. While that is true, there is a deeper reason—taking up macromolecules in a non-specific way would open the door for all kinds of viruses and Trojan horses. Extracellular digestion constitutes a firewall that excludes hazardous macromolecules.
Exceptions to the rule above are amoebas, which ingest not only macromolecules but even whole bacteria. However, the ingested bacteria remain confined within membrane vesicles called phagosomes, which get swiftly flooded with acid as well as aggressive chemicals and enzymes that kill and degrade the bacteria. The same occurs in our phagocytes, which are an essential part of our immune system (see slide 9.3.7).
| 1.6.2 |
The portal circulation |
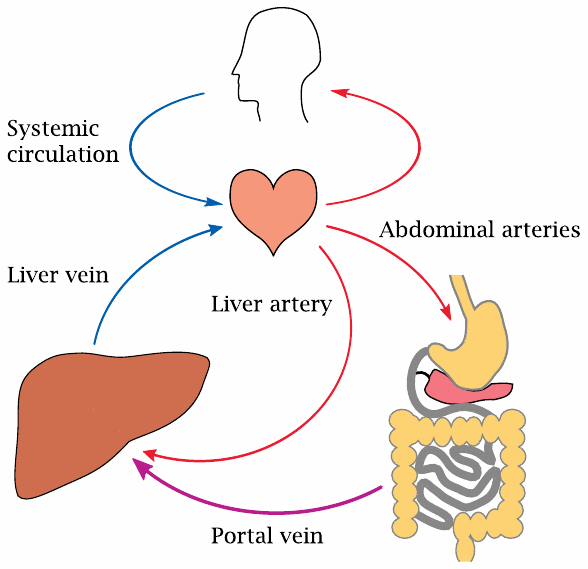
Upon uptake, most solutes will be exported on the other side of the mucosal cells and then find themselves in the blood stream. A peculiarity of the intestines is that all blood drained from them is first passed through the liver before being released into the general circulation. This serves a twofold purpose:
- 1.It gives the liver a chance to take excess amounts of substrates—glucose, amino acids—out of circulation and to store and process them. This serves to maintain stable blood nutrient concentrations, which is important for the well-being of the more sensitive and fastidious cells in the other organs.
- 2.The bacteria that reside in the large intestine produce ammonia and other toxic metabolites, which are cleared by the liver. In patients with liver failure, these toxic metabolites spill over into the systemic circulation, which among other things will lead to disturbances of cerebral function. The detoxifying activity of the liver also affects many drugs; the inactivation of drugs by the liver immediately following intestinal uptake is known as the first pass effect (see slide 19.1.3).
The large vein that drains all the blood from the intestines and channels it to the liver is the portal vein; together with its tributaries, it forms the portal circulation. Aside from the intestines, the pancreas and the spleen also have their blood drained into the portal vein.
In addition to the blood carried by the portal vein, which is at least partially oxygen-depleted, the liver also receives a direct supply of oxygen-rich blood through the liver artery. The two feeds branch out in parallel throughout the liver and eventually merge within the tissue of the liver lobules (see below), from which all blood is then drained toward the venous side of the general circulation.
| 1.6.3 |
Liver tissue structure |
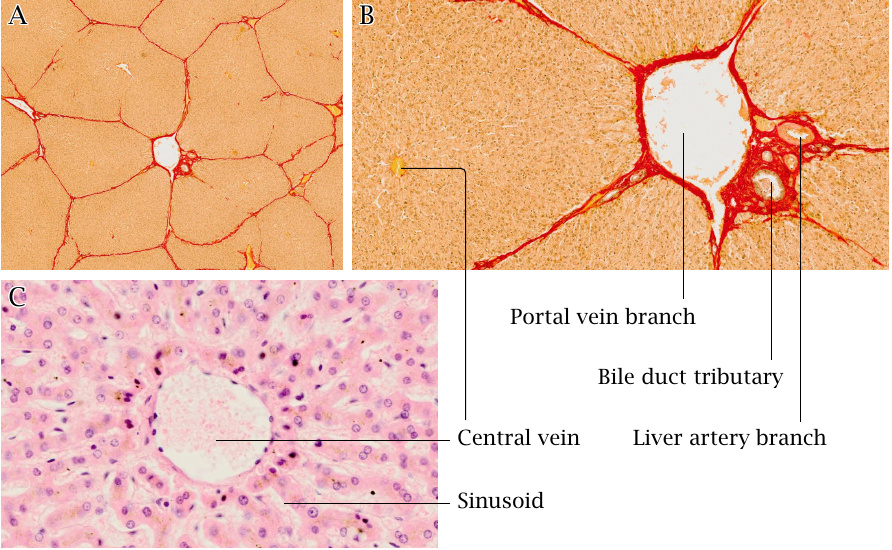
The liver has a peculiar tissue structure that is optimized for rapid and efficient solute exchange between the percolating blood and the liver cells. While in the tissues of most organs the blood is contained in capillaries with clearly defined boundaries and walls, the liver has a sponge-like structure that permits direct contact of the blood plasma with the liver cells.
A: The liver is organized into functional units called lobules, which measure ~2 mm across. In this tissue cross section, several lobules are demarcated by strands of connective tissue that are stained red.
B: Blood from branches of the portal vein and of the liver artery percolates each lobule and flows towards its central vein, which drains it into the general circulation. Bile duct branches drain bile from each lobule toward the intestine.
C: Higher magnification shows the sponge-like structure of the liver tissue. In life, blood flows through the sinusoids, which in this tissue section are visible as the voids between strands of liver cells. The intimate contact of the liver tissue with the percolating blood maximizes the rate of solute exchange between cells and blood plasma.
| 1.6.4 |
Blood flow and bile flow within the liver lobule |
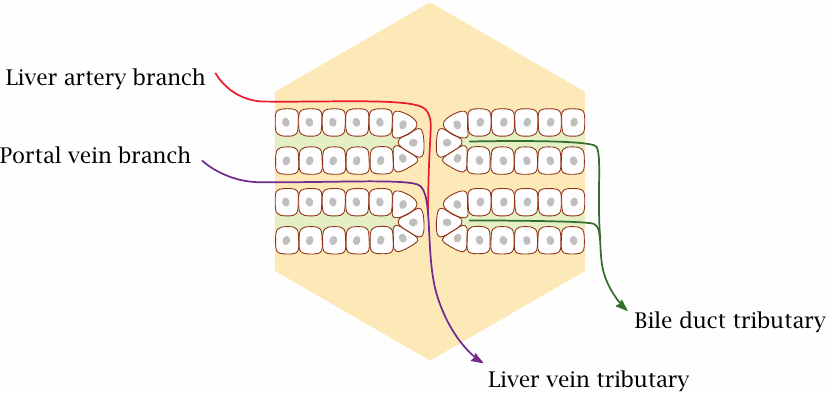
The epithelial cells in each liver lobule are arranged in parallel layers. The basolateral side of each cell faces the blood-filled sinusoid, while the apical side faces a bile duct tributary. These finest, uppermost bile duct branches are so thin that they can only be visualized using special histological techniques or by electron microscopy.
The liver cells extract solutes from the blood, modify them, and export them either back into the bloodstream or directly into the bile. This process is very efficient; with some solutes, extraction and modification is almost complete during a single pass through the liver.
| 1.6.5 |
The stomach: functions of gastric acid |
- HCl, pH 1–2
- secreted by specialized cells in the mucous membrane (parietal cells)
- kills germs contained in food; patients with lack of gastric acid are at increased risk of intestinal infection
- denatures food proteins and makes them accessible to cleavage by proteases
The activity of the HCl-secreting parietal cells is controlled by histamine H2 receptors; accordingly, H2 receptor blockers such as ranitidine are effective in the suppression of acid secretion. Another class of drugs used to the same end inhibit the ATP-dependent proton pump that actually brings about the secretion of acid.
Once upon a time, excessive secretion of gastric acid was considered the main cause of gastric and duodenal ulcers. We now know that that the true cause of ulcers is the bacterium Helicobacter pylori, and accordingly we treat this disease with antibiotics. Nevertheless, inhibitors of gastric acid secretion continue to be used as well, since gastric acid aggravates the ulcers and disturbs their healing.
Individuals that lack gastric acid, due either to a disease or to drugs that inhibit acid secretion, are more susceptible to orally contracted infectious diseases such as cholera, Salmonella enterocolitis, and intestinal tuberculosis.
| 1.6.6 |
Gastric acid and pepsin in protein digestion |
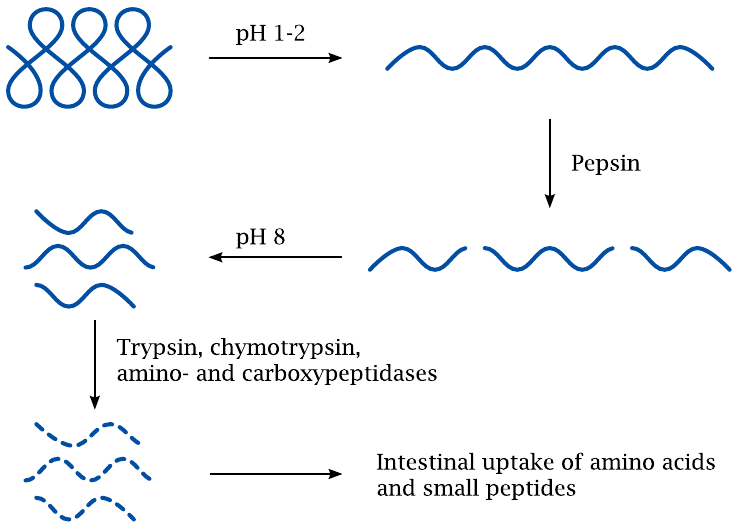
At very low pH, a protein molecule will become extensively protonated and thereby accumulate positive charges. The mutual repulsion of these positive charges will destabilize the protein and cause it to unfold. In this unfolded form, all the peptide bonds become exposed and accessible to proteases.
Protein digestion is initiated right away in the stomach by the protease pepsin, which is produced by the stomach mucous membrane. The peptide fragments will no longer refold, even after the pH has reverted to slightly above neutral values in the small intestine. Peptide digestion can therefore continue and be completed by the pancreatic proteases and peptidases encountered there.
While most proteins will be unfolded by gastric acid, there are exceptions; an obvious and important one is pepsin itself. Similarly, the coat proteins of many pathogenic viruses, for example poliovirus or hepatitis A virus, are fairly resistant to gastric acid as well. These viruses are therefore able to traverse the stomach intact and then infect the mucous membranes of the intestine.
| 1.6.7 |
Function of the exocrine pancreas |
-
secretion of digestive enzymes
- amylase
- proteases, peptidases
- lipases
- DNAse, RNAse
- secretion of sodium bicarbonate to neutralize gastric acid
The exocrine pancreas produces all the major depolymerizing enzymes for digestion. Therefore, if the pancreas is not working properly—often as a result of acute or chronic pancreatitis—maldigestion of all types of foodstuff results.
When the acidified food passes from the stomach to the duodenum, it is neutralized by copious amounts of sodium bicarbonate that is contained in the pancreatic juice, the bile, and the secretions of gland tissue embedded in the mucous membranes of the duodenum itself. Accordingly, the pH optima of the pancreatic enzymes are in the neutral to slightly alkaline range.6
| 1.6.8 |
Roles of bile in digestion |
- Bile acids solubilize triacylglycerol and make it accessible to pancreatic lipase
- Bicarbonate contributes to the neutralization of gastric acid
Among its many other functions, the liver also serves as an exocrine gland.7 The digestive juice secreted by the liver is known as bile and is rich in bile acids, which are important in solubilizing fat so as to render it accessible to enzymatic cleavage by pancreatic lipase.8 Bile that is not needed immediately is diverted to the bile bladder, where it is concentrated and stored. In the bile concentrate, solutes may exceed their solubility limit and start to precipitate or crystallize within the bile bladder, forming gallstones. This occurs most commonly with cholesterol and bilirubin, both of which are excreted with the bile (see chapters 11 and 17, respectively).
Like the pancreatic juice, the bile is also rich in sodium bicarbonate and contributes to the neutralization of the acidified stomach content as it enters the duodenum. Unlike the pancreatic juice, however, the bile does not contain digestive enzymes. Disruption of bile secretion will therefore cause deficient digestion of fat only, but not of proteins or carbohydrates.
The greater share of the bile acids is taken up again in the lowermost section of the small intestine, that is, the terminal ileum. Via the portal vein, they return to the liver, where they are extracted and again secreted.
| 1.6.9 |
The small intestine |
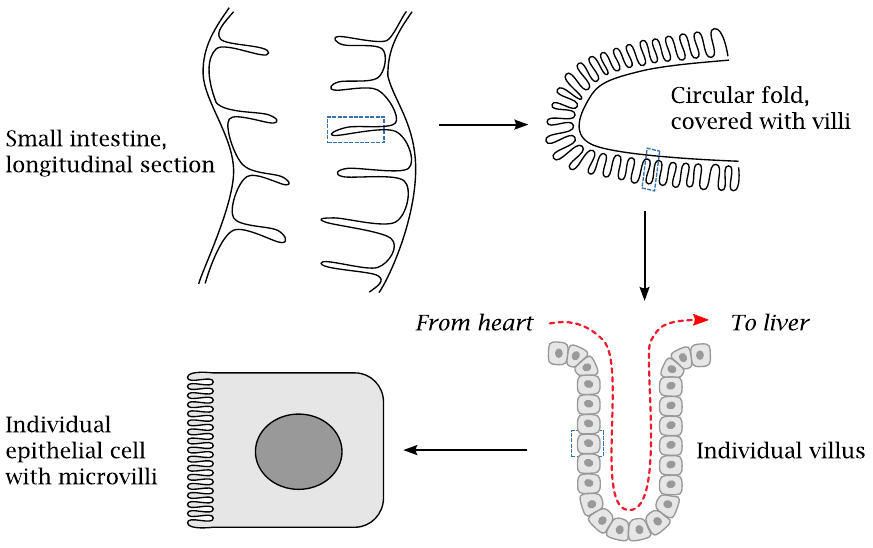
The small intestine comprises, from top to bottom, the duodenum, the jejunum, and the ileum. Small substrate molecules produced by the digestive enzymes within the gut are taken up by active transport across the mucous membrane of the small intestine. The capacity for substrate uptake is obviously related to the surface area. Accordingly, the mucous membrane is highly folded so as to maximize the surface available for substrate uptake. This slide illustrates how surface maximization is realized at all hierarchical levels of tissue and cell structure. The inner surface of the small intestine has circular folds, which in turn are covered by villi. The individual epithelial cells that cover the villi are, on their luminal surfaces, covered by microvilli.
The blood that perfuses the villi of the intestinal mucosa (red arrow) and carries away the absorbed nutrients is drained toward the liver via the portal vein (see slide 1.6.2).
| 1.6.10 |
Microscopic structure of the small intestine |
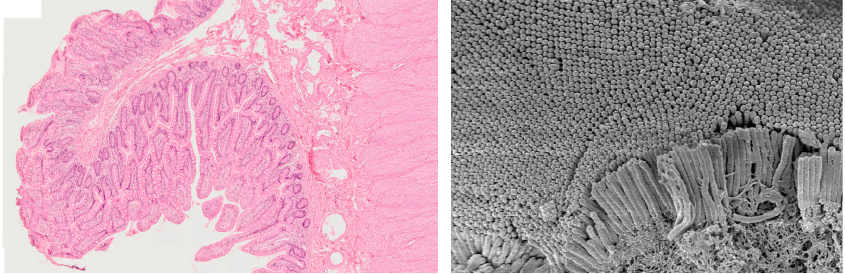
These microscopic pictures of the mucous membrane illustrate the villi and microvilli in the small intestine. The left panel shows a low-power view of a section across a circular fold, which is covered by a dense mane of villi. The right panel shows an electron-microscopic image of microvilli atop an individual epithelial cell. In combination, the circular folds, villi, and microvilli amplify the surface from approximately 0.3 to an estimated 30 square meters overall [1].
| 1.6.11 |
Amylose and amylopectin are polymers of α-d-glucose |
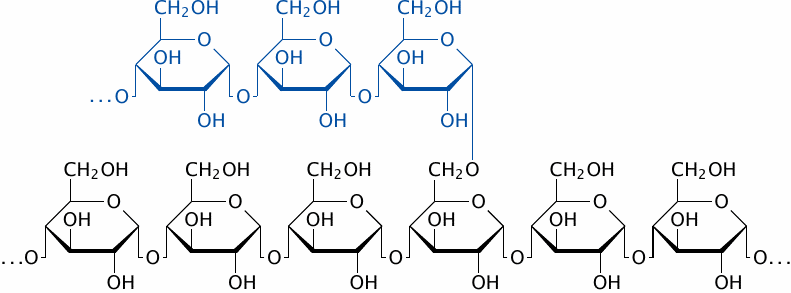
As an example of foodstuff processing in the small intestine, let us take a quick look at the digestion of starch. The constituents of starch are amylose and amylopectin. Amylose (black) is an unbranched α(1→4) polymer of d-glucose. Amylopectin additionally contains branches (blue) that are attached through α(1→6)-glycosidic bonds.
| 1.6.12 |
Amylase breaks down starch to maltose and isomaltose |
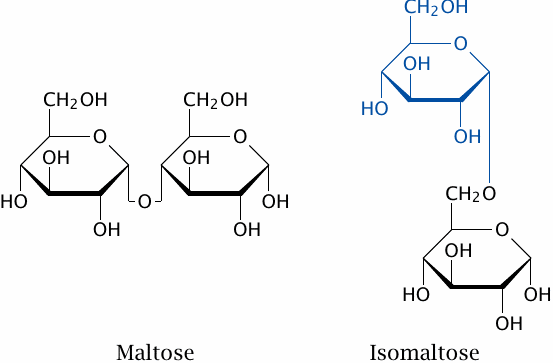
In the small intestine, amylose and amylopectin are broken down by pancreatic amylase. The main product is maltose, which is produced from amylose and from the linear α(1→4) stretches of amylopectin. Isomaltose originates from the α(1→6) branching points of amylopectin.
The two disaccharides are cleaved to glucose by maltase and isomaltase, respectively. These enzymes are anchored to the surfaces of the epithelial cells of the intestinal mucosa. The same epithelial cells then take up glucose by active transport (see next slide).
| 1.6.13 |
Mechanism of glucose uptake from the gut |
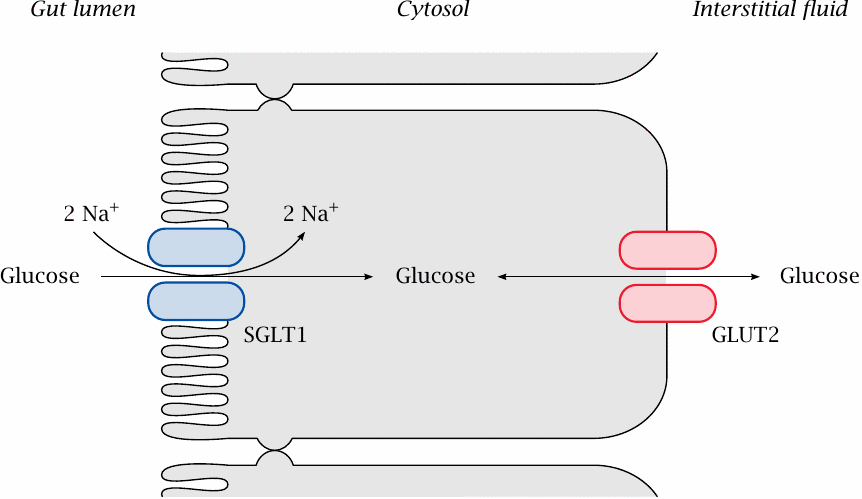
After digestion, the metabolites have to be taken up by the epithelial cells at the inner surface of the small intestine. In most cases, nutrients are taken up by active transport, which can transport solutes energetically uphill, that is, against their concentration gradients. Active transport thus enables the quantitative uptake of the nutrients.
In the case of glucose, active transport is driven by the simultaneous uptake of two sodium ions per molecule of glucose. This coupling is effected by the SGLT1 transporter. Sodium (secreted as bicarbonate) is plentiful in the gut lumen, while its concentration is low inside the cells. An additional driving force is the membrane potential: the cytosol is electrically negative relative to the extracellular space. The uphill transport of glucose is therefore driven by the simultaneous downhill movement of sodium. Similar transporters exist for other sugars, e.g. galactose, and for amino acids and nucleosides.
On the basolateral side of the intestinal epithelia—that is, the side that faces the surrounding tissue, not the gut lumen—glucose is released into the extracellular space, from where it can freely diffuse into the bloodstream to reach the liver. The export from the epithelial cells is mediated by GLUT transporters. These operate by passive transport, also known as facilitated diffusion (see slide 3.5.1). In other organs, GLUT transporters mediate the uptake of glucose. GLUT transporters are found in all cells of the body (see section 3.5).
| 1.6.14 |
The large intestine |
- Anaerobic milieu—99% of all bacteria in the large intestine are strict anaerobes
- Bacteria degrade non-utilized foodstuffs, reducing osmotic activity of gut content
- Mucous membrane recovers water and electrolytes
- Bacterial metabolism releases potentially toxic products (e.g. ammonia), which are taken up and inactivated by the liver
The cumulative volume of the fluids secreted into the stomach and the small intestine exceeds four liters per day. It falls to the large intestine to recover most of that fluid. This inevitably slows down the transport of the gut contents, which in turn will cause them to be overgrown with bacteria.9 The bacterial flora is mostly harmless, though, and it even helps with breaking down undigested remnants in the gut content and thereby freeing up the water bound osmotically by them. They produce some vitamins, too, for example folic acid, but also some potentially toxic substances such as amines and ammonia. The latter are taken up and dealt with by the liver.