| 7 |
Gluconeogenesis |
| 7.1 |
Introduction |
Glucose is a key metabolite in human metabolism, but it is not always available at sufficient levels in the diet. Therefore, a pathway exists that converts other foodstuffs into glucose. This pathway is called gluconeogenesis.
| 7.1.1 |
Glucose is an indispensable metabolite |
- The brain requires at least ~50% of its calories in the form of glucose
- Red blood cells exclusively subsist on glucose
- Glucose is a precursor of other sugars needed in the biosynthesis of nucleotides, glycoproteins, and glycolipids
- Glucose is needed to replenish NADPH, which supplies reducing power for biosynthesis and detoxification
These considerations make the need for gluconeogenesis quite clear—we can’t just leave the blood glucose level up to the vagaries of dietary supply.
| 7.1.2 |
Overview of gluconeogenesis |
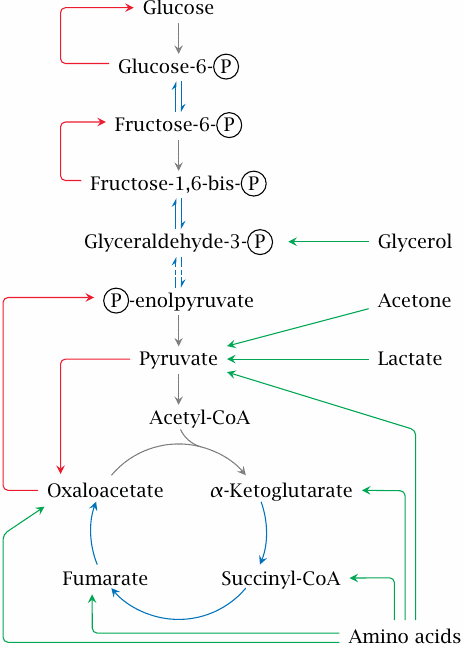
Gluconeogenesis is the reversal of glycolysis, with several workarounds for the irreversible reactions in that pathway. In this scheme, the reactions that are shared between glycolysis and gluconeogenesis are shown in blue, whereas reactions that are specific for gluconeogenesis are shown in red. As you can see, both pyruvate and oxaloacetate are starting points for red arrows; therefore, any pathway that yields either of these, or indeed any other intermediate of glycolysis, can supply substrate carbon for gluconeogenesis. These pathways are indicated here by green arrows.
The major substrate supply for gluconeogenesis is protein, both dietary and endogenous. Protein is first broken down into its constituent amino acids. Those amino acids that can be converted to pyruvate or any of the TCA cycle intermediates can serve as substrates for gluconeogenesis, and are therefore called glucogenic.
Leucine, lysine and the aromatic amino acids are degraded to acetyl-CoA or acetoacetate. Since acetoacetate is a ketone body, and acetyl-CoA can be converted to ketone bodies, these amino acids are called ketogenic. While it was believed for a long time that ketogenic amino acids cannot be converted to glucose in human metabolism, this is not strictly true, since the ketone body acetone can be converted to pyruvate (see slide 10.4.3). Nevertheless, the contribution of ketogenic amino acids to glucose regeneration is likely minor.
Gluconeogenesis proceeds only in the liver and the kidneys, and since the liver is five times larger than the two kidneys combined, it synthesizes most of the glucose. The pathway does not occur in the brain, fat tissue, or skeletal muscle. Together with glycogen degradation (see slide 8.3.5), gluconeogenesis ensures stable blood glucose levels between meals. Gluconeogenesis also enables us to maintain the necessary glucose levels when on a diet that is rich in protein but low in carbohydrates.
| 7.2 |
Reactions in gluconeogenesis |
Most reactions are shared with glycolysis, which we already know, and we here only need to consider the small number of reactions that are specific to gluconeogenesis.
The final reaction in glycolysis is the transfer of the phosphate group from phosphoenolpyruvate (PEP) to ATP. This reaction is irreversible because of the strongly exergonic nature of the accompanying rearrangement of pyruvate from the enol to the keto form (see slide 3.3.7). In gluconeogenesis, it takes two enzymatic steps to turn pyruvate back into PEP, namely (1)the carboxylation of pyruvate to oxaloacetate by pyruvate carboxylase, and (2)conversion of oxaloacetate to PEP by phosphoenolpyruvate carboxykinase.
| 7.2.1 |
The pyruvate carboxylase reaction |
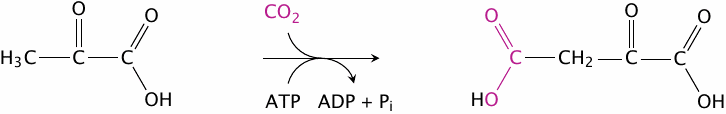
With the pyruvate carboxylase reaction, we are able to metabolically fix CO2—just like plants! Before we try to claim Kyoto treaty credits for this ability, however, it is necessary to consider that the very same molecule of CO2 gets released again in the next step. The whole purpose of transient CO2 fixation is to enable this subsequent reaction, which is shown in slide 7.2.4.
| 7.2.2 |
The active site of E. coli biotin carboxylase |
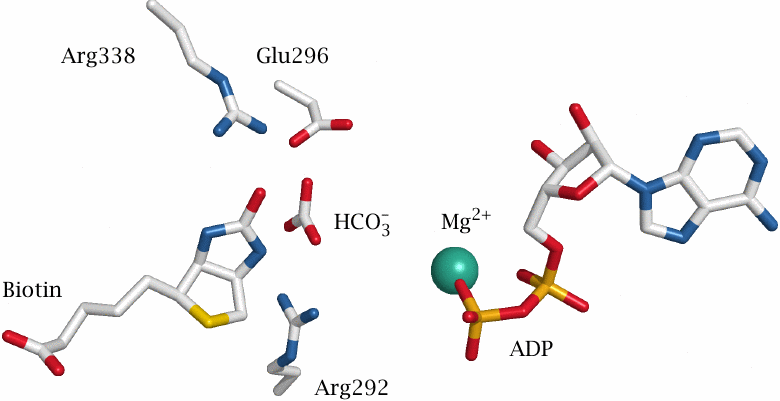
The pyruvate carboxylase reaction occurs in two separate steps, which in human metabolism are carried out in two distinct active sites of a single enzyme molecule. In E. coli, the two activities are found on separate enzyme molecules. The first enzyme activity is biotin carboxylase, which attaches CO2 to the coenzyme biotin.
The figure (rendered from 3G8C.pdb) shows the locations of the reactants, as well as of several strategic amino acid residues, within the active site of the E. coli enzyme. The reaction involves bicarbonate and ATP; the terminal phosphate group of ATP would fit into the space between ADP, arginine 292, and bicarbonate. The roles of arginine 338 and glutamate 296 are illustrated in the next slide.
| 7.2.3 |
Activation of bicarbonate and carboxylation of biotin |
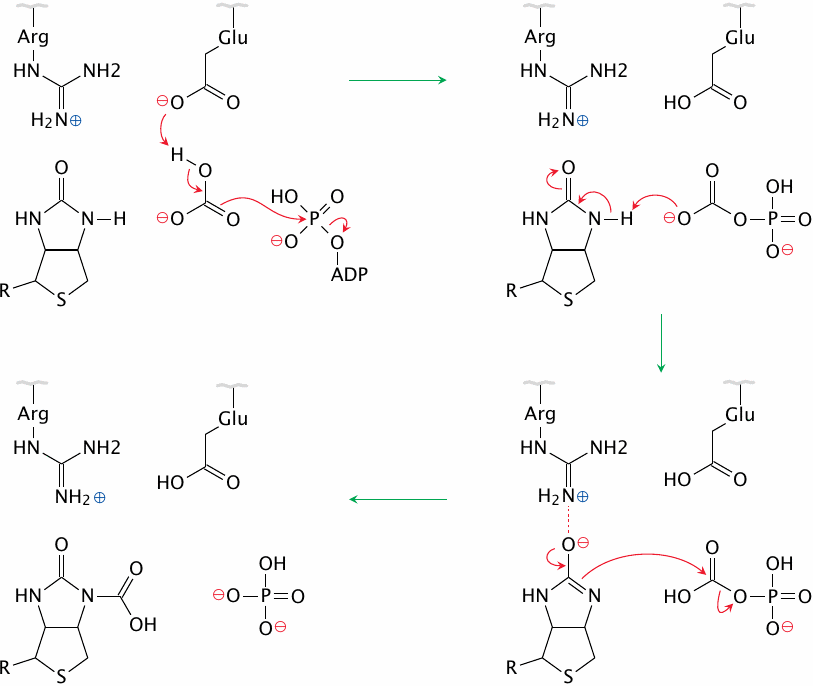
Glutamate 296 in the active site initiates the proceedings by deprotonating bicarbonate, which in turn attacks the terminal phosphate of ATP. This yields carboxyphosphate, which in turn deprotonates biotin; arginine 338 stabilizes the anionic biotin intermediate that forms transiently at this stage. The biotin anion finally attacks the carboxyphosphate, producing phosphate and carboxybiotin. Figure simplified after a scheme given in [29].
Beyond its role in biotin-dependent carboxylation reactions, carboxyphosphate (or carbonic-phosphoric anhydride) also occurs as an intermediate in the carbamoylphosphate synthetase reaction, which is the first step in the urea cycle (see slide 12.3.1).
| 7.2.4 |
The carboxylation of pyruvate |
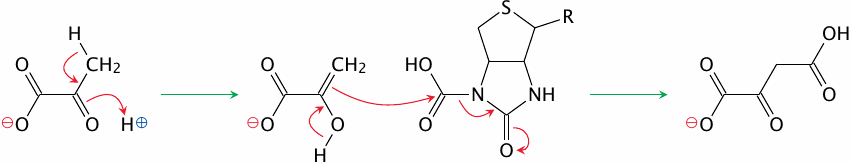
The second active site—or, in the E. coli pathway, the second enzyme—transfers the carboxyl group from biotin to pyruvate. The reaction begins with pyruvate adopting the enol configuration. The electrons of the C=C double bond then perform a nucleophilic attack on the carboxyl group, to which biotin readily yields. The product is oxaloacetate.
| 7.2.5 |
The phosphoenolpyruvate carboxykinase reaction |
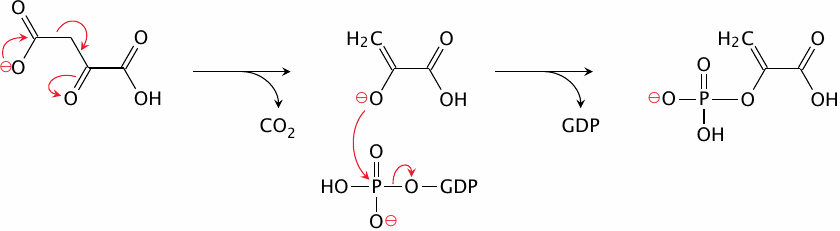
In the phosphoenolpyruvate carboxykinase reaction, the CO2 that just had been attached to the substrate leaves again. This gives rise to an enolpyruvate anion intermediate that attacks and acquires the terminal phosphate group of GTP. The product is phosphoenolpyruvate, which is an intermediate of glycolysis. All of the reactions between phosphoenolpyruvate and fructose-1,6-bisphosphate are reversible; we can therefore skip ahead to the latter metabolite.
| 7.2.6 |
Fructose-1,6-bisphosphatase and glucose-6-phosphatase |
| Fructose-1,6-bisphosphate + H2O | ⟶ | Fructose-6-phosphate + Pi | |
| Glucose-6-phosphate + H2O | ⟶ | Glucose + Pi |
These reactions revert the substrate phosphorylations that occur in the first and the third step of glycolysis, which are catalyzed by hexokinase and phosphofructokinase, respectively. In gluconeogenesis, the phosphate groups are simply hydrolyzed off, which is not a very difficult sort of reaction.
| 7.3 |
Energy balance of gluconeogenesis |
| Reaction | ATP/GTP input | ||
| 2 pyruvate | → | 2 oxaloacetate | 2 |
| 2 oxaloacetate | → | 2 PEP | 2 |
| 2 3-Ⓟ-glycerate | → | 2 1,3-bis-Ⓟ-glycerate | 2 |
| Total | 6 |
Gluconeogenesis requires an input of six equivalents of ATP or GTP for each molecule of glucose. In glycolysis, there was a net gain of only two molecules of ATP per molecule of glucose. The expenditure of an extra four equivalents of ATP in gluconeogenesis reverts the energy balance of the pathway, so that it actually proceeds in the opposite direction. Formation of no more than two ATP molecules makes it exergonic to turn glucose into pyruvate, whereas expenditure of six ATP equivalents makes it exergonic to turn pyruvate back into glucose.
| 7.4 |
Interactions of gluconeogenesis with other pathways |
As pointed out above (section 7.1.1), substrate carbon for gluconeogenesis accrues mostly from amino acid degradation and is harvested at the level of pyruvate or of TCA cycle intermediates. Pyruvate carboxylase, which turns pyruvate into a TCA cycle intermediate, is important not only in gluconeogenesis, but also in the replenishment of TCA cycle intermediates, which may become depleted through diversion to the biosynthesis of amino acids or of heme. Therefore, this enzyme is expressed not only in the organs that perform gluconeogenesis (liver and kidneys) but ubiquitously.
Gluconeogenesis is also part of two interorgan cycles, namely, the Cori cycle and the glucose-alanine cycle. These will be discussed after several other participating pathways have been introduced (see slides 8.5.3 and 12.3.5).
| 7.4.1 |
Mitochondrial substrate transport in gluconeogenesis |
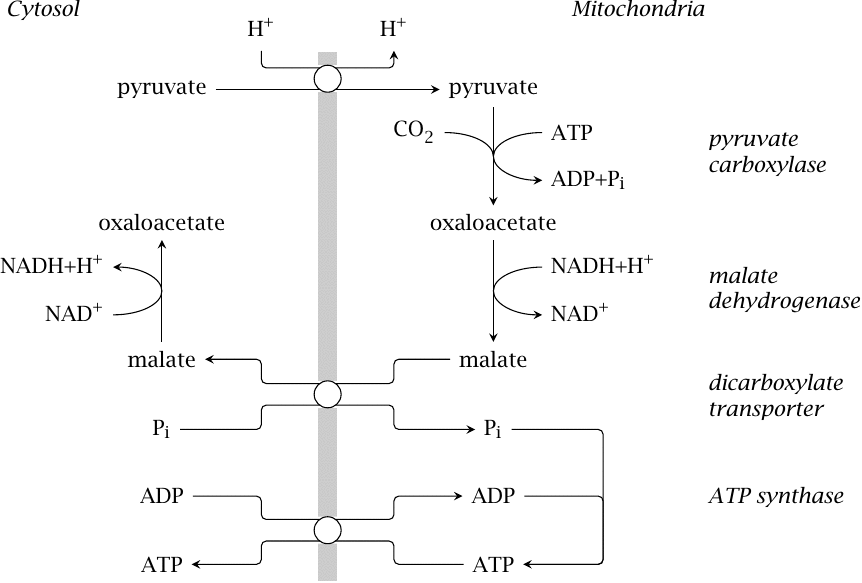
In keeping with its role in replenishing TCA cycle intermediates, pyruvate carboxylase resides inside the mitochondria. The next step in gluconeogenesis, catalyzed by phosphoenolpyruvate carboxykinase, occurs in the cytosol; therefore, oxaloacetate must in some way be exported from the mitochondria again.
We had already seen that the mitochondrial concentration of oxaloacetate is low, and that the malate dehydrogenase equilibrium favors malate (section 5.5). It turns out that substrate export occurs indeed at the level of malate, which is exchanged for phosphate by a mitochondrial transport protein known as the dicarboxylate carrier. The malate dehydrogenase reaction is then reversed in the cytosol; the NADH produced can be used in the reversal of the glyceraldehyde-3-dehydrogenase reaction (see slide 3.3.5), which occurs later on in gluconeogenesis.
The phosphate that entered the mitochondrion in exchange for malate can be used by ATP synthase, and the ATP be exchanged for cytosolic ADP, which balances the entire transport cycle and supplies one ATP to the cytosol, where it may for example be used by phosphoglycerate kinase in gluconeogenesis.
The dicarboxylate carrier that exports malate to the cytosol is susceptible to inhibition by methylmalonate. The coenzyme A thioester of methylmalonate occurs in the metabolic utilization of fatty acids with uneven numbers of carbon atoms (see slide 10.3.6). This pathway requires vitamin B12. A lack of the vitamin will cause free methylmalonate to accumulate. This may inhibit gluconeogenesis and thus account for the clinical hypoglycemia that sometimes accompanies vitamin B12 deficiency [30].
| 7.4.2 |
Ethanol degradation inhibits gluconeogenesis |
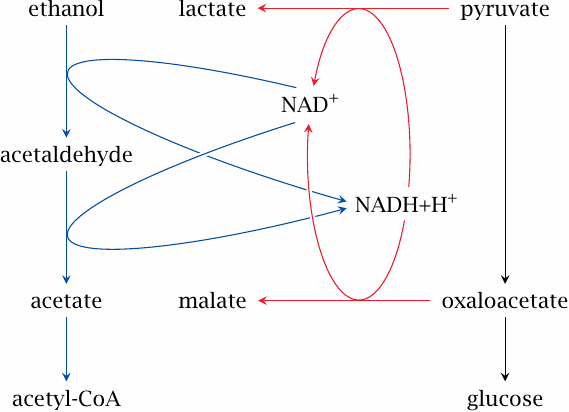
Like gluconeogenesis, ethanol degradation occurs in the liver. The utilization of one molecule of ethanol by alcohol dehydrogenase and then aldehyde dehydrogenase yields acetate, which is converted to acetyl-CoA by acetate thiokinase.
For each molecule of ethanol degraded, two equivalents of NAD+ are reduced to NADH. This raises the cytosolic [NADH]/[NAD+] ratio, which in turn reduces both pyruvate and oxaloacetate and thus deprives gluconeogenesis of its substrates. In alcoholic patients, this problem is often compounded by a low intake of carbohydrates. Clinically manifest hypoglycemia with unconsciousness is a well-known and potentially dangerous complication in alcohol addiction.
| 7.5 |
Regulation of gluconeogenesis |
The activity of enzymes in gluconeogenesis is regulated by several mechanisms according to the metabolic needs of the cell and those of the entire body.
| 7.5.1 |
Simultaneous activity of glycolysis and gluconeogenesis creates futile cycles |
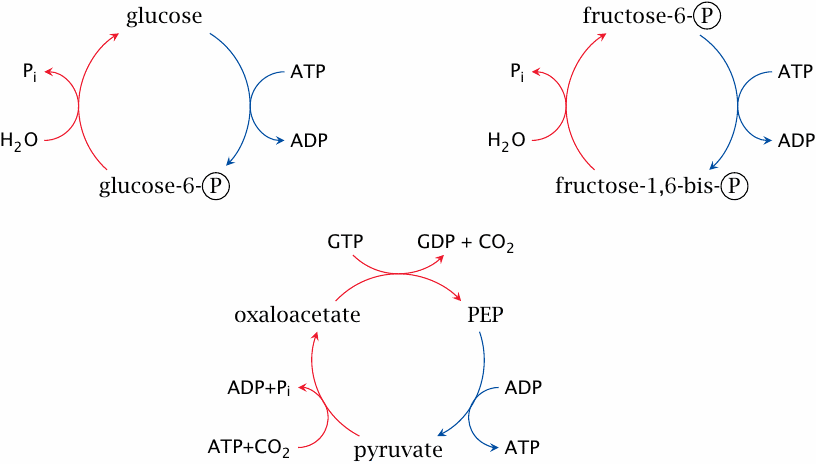
Each of these short cycles combines one enzyme from glycolysis (blue arrows) with one or two enzymes from gluconeogenesis (red arrows). In each case, the net result of the cycle is simply the consumption of ATP or GTP and the release of heat.
These cycles have been shown to run in living cells at appreciable levels, possibly for the sake of heat production; and as pointed out in chapter 2, such substrate cycles also sharpen up regulatory responses (see slide 2.5.7). Nevertheless, their activity must be kept in check in order to avoid excessive wastage of ATP. Some, but not all of the regulatory mechanisms that exercise this control are understood.
| 7.5.2 |
Glucose phosphorylation cycling involves two separate compartments |
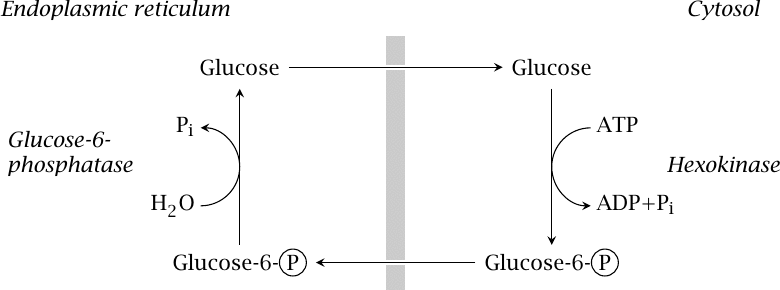
In this cycle, hexokinase or glucokinase operates in the cytosol, whereas glucose-6-phosphatase is located in the endoplasmic reticulum. It seems likely that flow through the cycle is limited by the capacity of glucose-6-phosphate transport to the ER. Interestingly, the transporter and the phosphatase are expressed in many tissues, not just those that perform gluconeogenesis [31]; it is not clear at present what, if any, function other than futile cycling this might serve.
| 7.5.3 |
Allosteric regulation limits fructose-6-phosphate phosphorylation cycling |
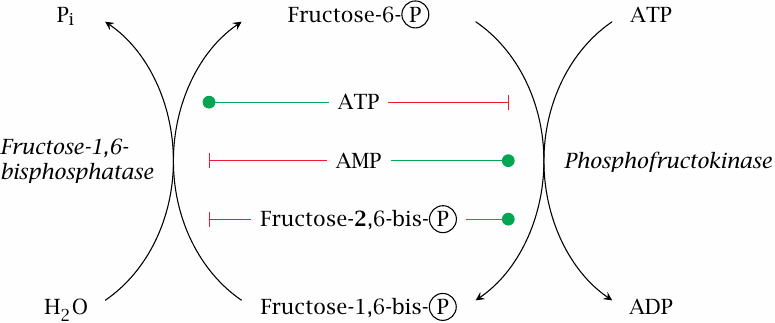
Phosphofructokinase is inhibited by ATP. This makes sense, since ATP formation is the main purpose of glycolysis, in conjunction with the TCA cycle and the respiratory chain. On the other hand, depletion of ATP will result in a buildup of ADP and AMP; it thus also makes sense that ADP and AMP stimulate phosphofructokinase (section 2.5).
Fructose-1,6-bisphosphatase is stimulated by ATP and inhibited by AMP. This behavior is opposite to that of phosphofructokinase, and it ensures that only one of the two enzymes will be fully active at any given time, which will help to limit the rate of futile cycling.
| 7.5.4 |
The level of fructose-2,6-bisphosphate is controlled by hormones |
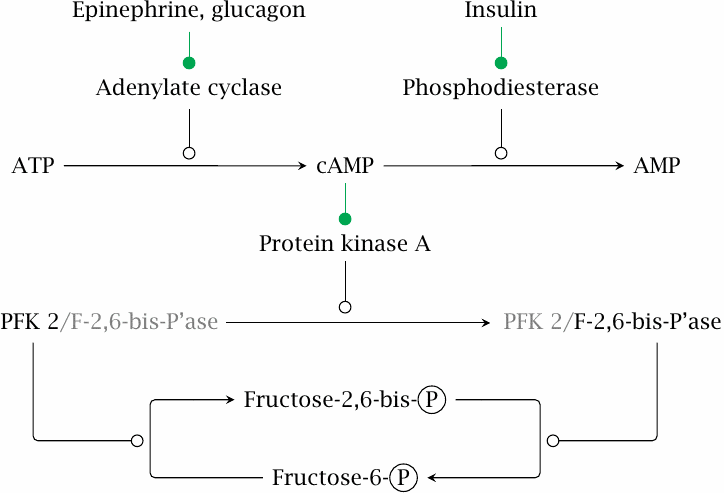
Phosphofructokinase and fructose-1,6-bisphosphatase respond in opposite manner to a third allosteric effector, namely, fructose-2,6-bisphosphate. This molecule is not a regular metabolite of glucose metabolism but is synthesized solely for the sake of its regulation, and it occurs at much lower levels than fructose-1,6-bisphosphate. Its concentration is under the control of hormones via the secondary messenger 3’,5’-cyclo-AMP (cAMP). The entire cascade consists of the following stages:
- 1.The hormones bind to their respective receptors on the cell surface, which then promote the formation of cAMP by adenylate cyclase (glucagon and epinephrine) or its degradation by phosphodiesterase (insulin).
- 2.cAMP binds to and activates protein kinase A, which phosphorylates the enzyme phosphofructokinase 2 (PFK 2). This phosphorylation can be reversed by a protein phosphatase.
- 3.PFK 2 has two opposite activities: In the dephosphorylated state, it acts as the kinase and therefore raises the level of fructose-2,6-bisphosphate. In the phosphorylated state, it acts as the corresponding phosphatase and therefore decreases the level of fructose-2,6-bisphosphate.
Since fructose-2,6-bisphosphate activates phosphofructokinase and at the same time inhibits fructose-1,6-bisphosphatase, the upshot of glucagon and epinephrine action is to promote gluconeogenesis and inhibit glycolysis. Insulin has the opposite effect. The actions of all these hormones will be considered in more detail in chapter 13.
| 7.5.5 |
The secondary messengers cAMP and fructose-2,6-bisphosphate |
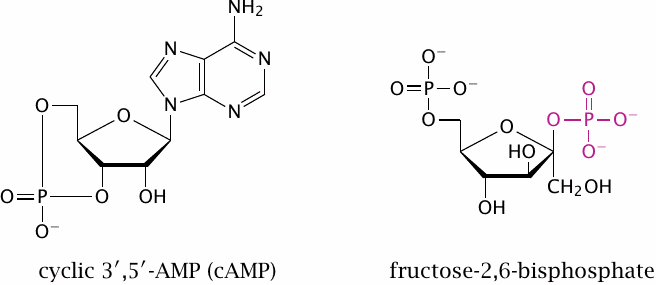
This slide just shows the structures of the secondary messengers introduced in the previous one. In the structure of fructose-2,6-bisphosphate, the phosphate group attached to the 2′-position is highlighted.
It should be noted that, among the three allosteric effectors of phosphofructokinase and fructose-1,6-bisphosphatase, ATP and AMP reflect the energetic situation of the cell itself, whereas the level of fructose-2,6-bisphosphate is regulated by hormonal stimulation and thus controls the same enzymes on behalf of the needs of the body as a whole. This is a nice example of how regulatory signals of different origin and meaning are integrated at the molecular level.
| 7.5.6 |
Regulation of pyruvate kinase |
- allosteric activation by fructose-1,6-bisphosphate
- allosteric inhibition by ATP and alanine
- inhibition by PKA-mediated phosphorylation
In the third cycle shown in slide 7.5.1, throughput is limited between PEP and pyruvate. The liver form of pyruvate kinase is allosterically activated by fructose-1,6-bisphosphate. It is allosterically inhibited by alanine and by ATP, and it is also inhibited through phosphorylation downstream of cAMP.
The effects of ATP and fructose-1,6-bisphosphate provide some more examples of feedback and feed-forward regulation. Alanine is a key substrate for hepatic gluconeogenesis, since it carries substrate carbon and surplus nitrogen from muscle to the liver in catabolic conditions (see slide 12.3.5); it thus makes sense that alanine should inhibit glycolysis.
Also note that this cycle involves both mitochondrial and cytosolic enzymes, and some of the mitochondrial transporters involved (see slide 7.4.1) might also constrain the flow through this cycle.