| 4 |
Early measurements of residual radioactivity |
This chapter examines reports on early field measurements in Hiroshima and Nagasaki by American and Japanese investigators. It concludes that the very limited evidence available does not substantiate the high initial levels of radioactivity on the ground that are implied by the conventional story of the nuclear bombings.
As explained in Section 2.10, most of the radiation produced by a nuclear bomb is released at the time of the detonation in the form of γ-rays and neutrons. Both can in principle be monitored in real time with suitable detectors [36], and the means were already available in 1945. The γ-radiation, in particular, should have been picked up by X-ray dosimeters, of which several types were already known in the 1940s [72], and at least the more modern hospitals in Hiroshima should have been equipped with them. I have not seen any reports of X-ray dosimeter readings that were taken during the bombing, but of course at that instant nobody had reason to suspect that an atomic bomb had been dropped. The upshot is that we have no record of an immediate, quantitative measurement of the radiation released during the blast.
In the absence of such direct measurements, one can try to reconstruct the radiation intensity during the detonation from indirect measurements of induced radioactivity (Section 2.10.3) and of thermoluminescence (Section 2.8.3). Here, we will consider measurements of radioactivity that occurred on-site in the days and weeks directly after the bombing. These measurements used Geiger counters or similar devices that could not identify radioactive isotopes, which also implies that they could not distinguish between the fission products carried by radioactive fallout and induced radioactivity. They are nevertheless of great value, since both fallout and induced radioactivity comprise mixtures of isotopes with very different half-lives. The very short-lived isotopes would produce high intensity beginning with the detonation but would drop to insignificant levels after time intervals ranging from hours to weeks; thereafter, the much slower decay of the longer-lived isotopes would sustain a residual activity somewhat above the natural background for several months to years (see Section 2.3.1). Thus, a high initial level of radioactivity which then rapidly drops by several orders of magnitude would be characteristic of a nuclear detonation. On the other hand, absence of the initial short phase of high activity would indicate that no such detonation had occurred.
| 4.1 |
Timeline and findings of early field measurements |
Given the great potential value of early measurements, there is a striking shortage of actual data. The 235U bomb supposedly dropped upon Hiroshima had never been tested before, and it has never been used again. Under these circumstances, one surely would expect that the Americans would have started their investigations at the earliest opportunity after the Japanese surrender; in fact, already before the surrender they might have advised the Japanese of the best ways to ascertain the nature and effect of the weapon. They might even have asked a neutral third party to assist the Japanese with the investigation, which would have been in the best interest of both sides. However, it seems the Japanese received no such assistance. Even more strikingly, after the capitulation it still took the Americans several weeks to send even some small advance parties of investigators; not before October did the Manhattan Engineers begin their own measurements (see Table 4.1).54 Neither did they make up for lost time afterwards. The American physicist Robert Wilson, writing on the bomb radiation in 1951, began by summing up the state of this research [73]:55
It is no simple problem to determine the X-ray and neutron dosages which were received at Hiroshima and Nagasaki. Much of the meager primary data that were written down has been dispersed or lost—that which existed but was not written down is mainly forgotten.
| Team/University | Date | Location and findings |
| Osaka | August 11th | up to 5 times natural background several hundred meters from the hypocenter |
| Kyoto | August 11th | up to 10 times background several hundred meters from hypocenter |
| Kyoto | August 15th/16th | 6 times natural background at Asahi bridge, otherwise weak activities |
| RIKEN | August 17th to October 20th | Imanaka [75] reports only relative readings; values in August and October are of similar magnitude |
| RIKEN | September | activity up to 6 times above background in fallout area |
| RIKEN | October 1-22 | activity up to 9 times above background near hypocenter |
| RIKEN | January 1946 | activity 6 times above background near hypocenter, 3 times background fallout area |
| Manhattan Engineers | October 1945 | activity up to 15 times above background near hypocenter, up to 8 times in fallout area |
| Hiroshima | 1948 | activity up to 2.5 times background in fallout area |
A timeline of early measurements, by both Japanese and American investigators, is given by Imanaka [75]. Table 4.1 provides a summary. While there is some variation in the results, all measurements agree that the level of activity is above the natural background but overall quite low, certainly nowhere near the levels required to induce acute radiation sickness (see Chapter 8). Among these results, the most important are those of August 11th, since they were obtained just five days after the bombing, and thus within a time period during which there should still have been substantial activity from short-lived isotopes.56 This is illustrated in Figure 4.1A, which shows the hypothetical time course of induced radioactivity at the hypocenter, for the first three months after the bombing. The shape of this curve has been inferred from later experiments, in which soil samples from Hiroshima were irradiated with neutrons, and the activities of the major isotopes produced by neutron capture were measured.57 The height of the curve was calibrated to a single reported measurement, which was taken 87 days after the bombing; according to Ishikawa et al. [8], and in keeping with the general trend evident from Table 4.1, this measured value amounted to ten times the natural background.
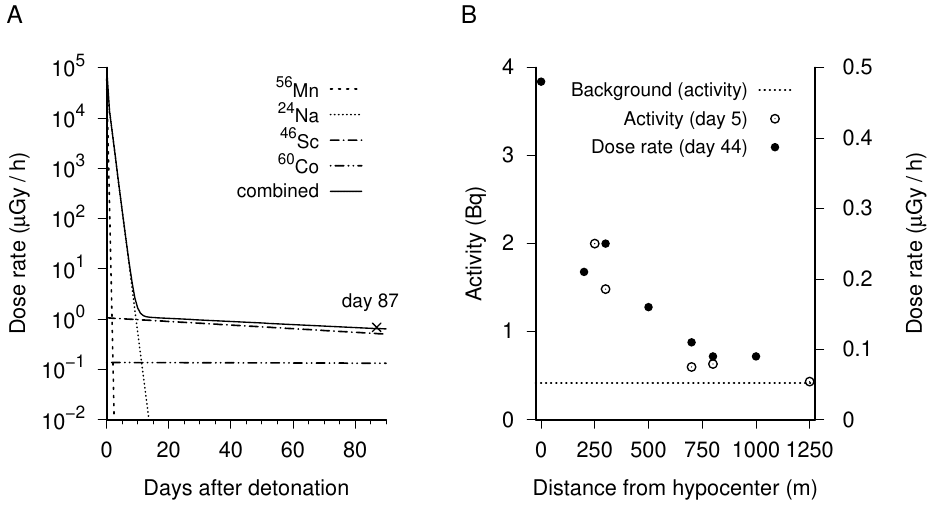
It is evident that the estimated activity changes very little after the 15th day. On the other hand, measurements within the first week should have shown a much higher activity. The question then is: did they? It seems that activity at the hypocenter was not measured within that time frame,58 however, we can estimate it by comparing the two data series shown in Figure 4.1B. These data were collected at 5 days and 44 days, respectively, after the detonation. The measurement on day 5 is scaled in units of activity (Bq, or decays per second), while the measurement on day 44 is given in units of absorbed energy dose (µGy).59 They have been overlaid and scaled to show that they vary similarly with the distance from the hypocenter, as they should. We can use this similarity to estimate that the activity at the hypocenter on day 5 would have been approximately 4 Bq, which is some ten times above the natural background. The single measured data point in panel A, at 87 days, was also about 10 times above the natural background [8]. Thus, while the neutron activation experiment shown in panel A indicates that between day 5 and day 87 the level of radioactivity should have decreased by a factor of 100, the observed factor is 1—that is, no decrease has occurred. Even though both of these factors are approximations, they cannot be reconciled; one must be false.
| 4.2 |
Shimizu’s sulfur activation measurements |
Against the various accounts of weak observed activity throughout the early period, one report stands out—that by Sakae Shimizu [37], one of the researchers from Kyoto University who undertook several expeditions to Hiroshima in August and September (see Table 4.1). The key pieces of his evidence are a magnetic piece of iron, a horse bone, and three porcelain insulators containing sulfur. When these samples were examined for β-radiation in the laboratory at Kyoto, all showed significant activity, which Shimizu ascribes to activation by neutron capture. Of particular interest is the activation of sulfur, since it requires highly energetic (fast) neutrons [36], which unlike those of low energy would be expected in a nuclear bomb but not in the natural background radiation.
There are strong reasons, however, to reject Shimizu’s evidence. Both with his sulfur samples and those reported by investigators from RIKEN [79, p. 216], the activity as a function of distance from the hypocenter is physically implausible. This will be discussed in detail in Section 6.3.1.
Another reason to doubt Shimizu’s sulfur activation data is that this line of evidence was not pursued any further. Activation of sulfur (32S) would have been singularly useful to determine the strength and exact location of the detonation, as well as the reach of the fast neutrons produced by it. The activation of sulfur produces radioactive phosphorus (32P), which has a half-life of 14.3 days. Thus, if Shimizu’s early high readings had been correct, enough activity should have remained even at 4-6 weeks after the detonation, that is, long enough for the Americans to carry out their own measurements. There is, however, no indication that they ever did so.60
Suspicion is also warranted concerning Shimizu’s piece of radioactive iron. It is said to have shown and activity of 374 cpm, or approximately 6 Bq. On its own, this is not problematic. However, the sample is said to have been “buried in a collapsed house near the hypocenter”, and furthermore to have consisted of a “horseshoe magnet of an integrating Watt-meter.” Such instruments are surely much more commonly encountered in physics laboratories than in urban dwellings. Furthermore, if indeed the house containing this instrument had collapsed, how could this sample have been discovered afterwards? Its rather weak radioactivity could not possibly have been detected from above the pile of rubble covering it—particularly if we believe that so soon after the bombing the whole place was brimming with radioactivity.
Among all of Shimizu’s samples, the highest activity is reported for a horse bone. This activity is attributed mostly to the activation of phosphor by the capture of slow neutrons. In Chapter 6, it will be shown that collectively the phosphor activation measurements are inconsistent with those pertaining to sulfur activation. Overall, therefore, not one of the findings reported by Shimizu can be taken at face value.
| 4.3 |
Conclusion |
Once we disqualify Shimizu’s findings, two major conclusions emerge. The first one is that, among all measurements on the ground, only those that occurred in the first week have any real power to confirm or refute a nuclear detonation; and their consistently low levels of activity clearly refute it.
The second conclusion is simply that which was already spelled out by Wilson [73], namely, that both the acquisition and the documentation of early radioactivity measurements were wholly inadequate. This inadequacy speaks louder than the evidence itself. If the official story had been true, if the bomb had indeed been the world’s very first 235U bomb, such obviously willful negligence would be inexplicable. Fantastic amounts of work and treasure had been poured into the development of this revolutionary weapon; surely those who had accomplished it would also want a detailed record of the outcome and proof of their success. If, on the other hand, the official story were indeed a lie, then the neglect would be entirely understandable, since richer and more detailed evidence would only increase the chances that the fraud might be uncovered in the end.