| 3 |
The nuclear fallout at Hiroshima and Nagasaki |
The radioactive fallout of the Hiroshima bombing, while weak, can be unequivocally detected. Its isotopic composition, however, indicates that it was not caused by the detonation of a 235U bomb, but instead by the dispersal of reactor waste.
At Nagasaki, a high activity of plutonium is found in the sediments of a reservoir near the city. However, a stratigraphic study of these sediments shows that the plutonium entered the reservoir some time after the bombing; this agrees with the assessment by ‘Manhattan Project’ scientists, initially classified, that purification of plutonium had not yet been achieved in 1945. Moreover, the ratio of 239Pu to 137Cs contained in the sediment does not correspond to the purported fission yield of the Nagasaki bomb.
Collectively, the findings presented in this chapter suffice to conclusively reject the official story of the atomic bombings.
It is commonly believed that, while the atomic bombs in Japan exploded with unprecedented force, they were dwarfed by the much more powerful ones that were developed and tested in subsequent decades. According to Carter [54], the nuclear bomb tests during the 1950s, 60s, and 70s totaled 905 in number and 344 megatons in yield. Collectively, these tests produced a large amount of radioactive fallout, much of which was dispersed all over the Northern hemisphere, and which can be ubiquitously detected with modern, sensitive instruments.
If we want to determine how much fallout remains at Hiroshima and Nagasaki from the original bombs, we must distinguish it from the ubiquitous global fallout. There are two ways of doing so. Firstly, we can look for samples that were secured, or at least protected, early on, before they could become contaminated with the global fallout. Secondly, we can exploit the distinct nature of the purported Hiroshima bomb, which used highly enriched 235U as its fuel, while the Nagasaki bomb, as well as the great majority of all later bomb tests used plutonium (239Pu) instead.40
The fission products which form from 235U and 239Pu are quite similar; in particular, the widely used fallout tracer 137Cs is found with both. However, unfissioned 235U itself, when exceeding the natural isotope ratio relative to 238U, would be a specific tracer for the Hiroshima bomb. The study by Shizuma et al. [6] cited earlier applied both of these principles: it quantified both 235U and 238U in samples touched only by local but not by global fallout. This circumstance earned it preferred treatment.
| 3.1 |
Uranium isotopes in soil samples |
Apart from 235U and 238U, several other uranium isotopes exist that have low abundance, yet can be of value in understanding what did or did not happen at Hiroshima. Sakaguchi et al. [56] examined the abundance of 236U, which forms from 235U by neutron capture without fission. A complicating factor, however, is that 236U also arises through radioactive decay of 240Pu, the second most abundant plutonium isotope. Since 236U decays very slowly and therefore has low specific activity, the method used in this study was mass spectrometry.
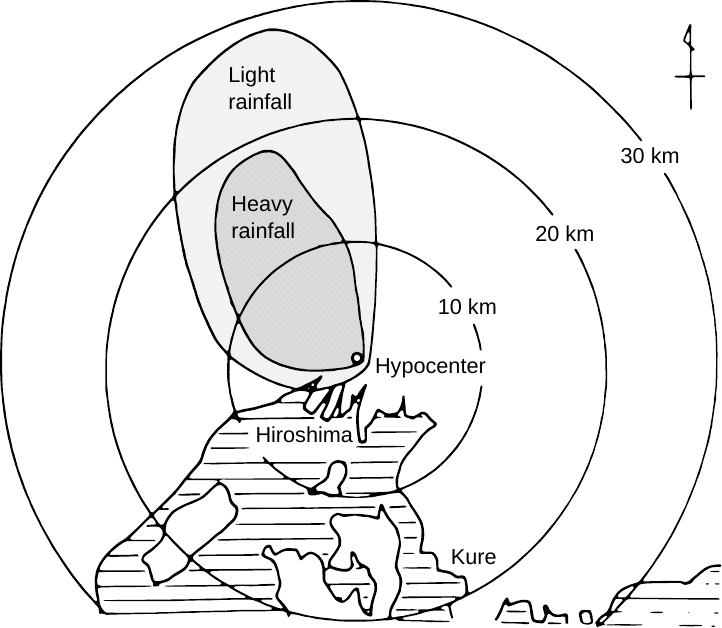
Starting from conventional estimates of bomb size, degree of 235U enrichment, and fission yield, the authors estimate that 69 g 236U should have been generated in the detonation, and they set out to look for it in the area affected by the black rain (see Figure 3.1).41 At this point, you might not be surprised to learn that they do not find it; or more accurately, they do find some 236U, but after comparison with plutonium levels and with samples from a control area in Japan taken to be unaffected by ‘Little Boy’, they conclude that all of it must be attributed to the global fallout. To explain the lack of a discernible local contribution, they assume that the black rain transported only a very small fraction of the radioactive matter generated in the blast.42
The major component of natural uranium, 238U, undergoes α-decay, which is followed rapidly by two successive β-decays; this yields 234U. The half-life of 238U is very long (4.47 billion years), whereas that of 234U is comparatively short (246,000 years). At steady state, 234U will decay exactly as fast as it is formed through decay of 238U (see Section 2.3.3). Therefore, if we stick a sample of natural uranium into a radiation counter, we should measure equal activities for these two isotopes. The relation should be different, however, with enriched uranium, as was supposedly used in the Hiroshima bomb. Because 234U is close to 235U in atomic weight, both isotopes should have been enriched together relative to 238U. Assuming that in the Hiroshima bomb 234U, like 235U, was enriched by a factor of about 100 over its natural abundance, whereas 238U was reduced by a factor of 5, the activity (but not the abundance) of 234U in the bomb material should exceed that of 238U by some 500 times. Therefore, the 234U /238U activity ratio should be a very sensitive probe for the detection of residual bomb uranium.
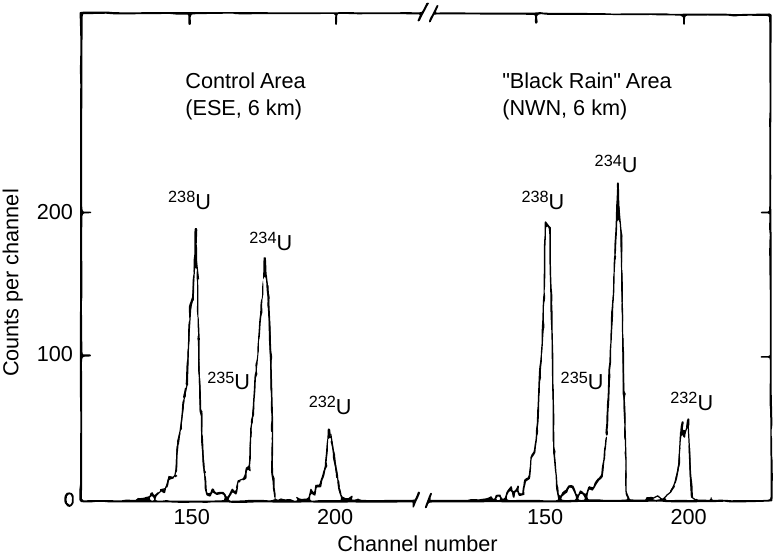
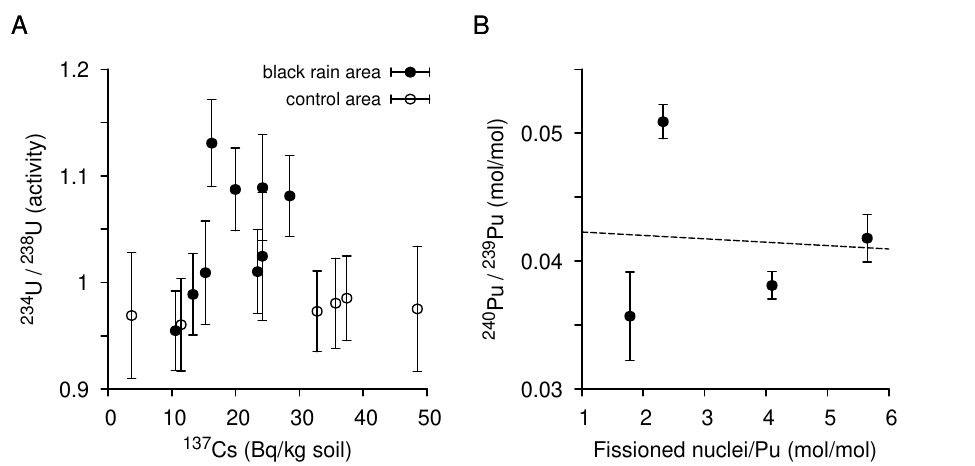
A very careful study that employed this probe was carried out by Takada et al. [57]. The samples consisted again of soil from the black rain area. What makes this study particularly interesting is the attempt to chemically separate bomb-derived uranium from that which constitutes the natural background. The bomb fallout should only adhere to the surface of the soil mineral particles, whereas the natural uranium should mostly reside within them. Thus, to extract the fallout, the soil samples were gently leached with dilute acid, which should strip only a shallow, superficial layer from the particles; the background was then recovered by dissolving the residue with concentrated acid.
In the fraction recovered with dilute acid, 234U activity indeed exceeded that of 238U—but only by a factor of approximately 1.15; compare this to the factor of about 500 expected for pure, highly enriched bomb uranium. This slight excess was observed only with samples from the black rain area, but not with those from a control area outside it.43 The activity of 235U, which in pure bomb-uranium should exceed that of 238U some 25 times, remained very low in all samples (see Figure 3.2).
As with the study by Shizuma et al. [6] cited before (Section 1.2), we have evidence of a small yet distinct deviation from the natural uranium isotope distribution; and the magnitude is similar between the two studies. There are two explanations in principle—namely, either that a minuscule amount of highly enriched bomb uranium was diluted to near nothingness by natural background, or that the degree of 235U enrichment in the dispersed artificial material was much lower than announced. Takada’s failure to detect a higher degree of enrichment even when taking steps to concentrate the bomb uranium clearly militates in favor of the second alternative.
Considering this evidence, as well as the state of technology which then prevailed (see Section 3.6 below), I feel certain that no highly enriched 235U was released at Hiroshima. However, here is how to prove me wrong: obtain a sample of pristine glacier ice, and analyze it for 235U and 238U. This has been done for both cesium and plutonium on a sample from Ellesmere Island in the Canadian arctic, and it is claimed that the imprint of the Nagasaki bomb is detectable in the layer of ice that was deposited in the year 1945 [58]. Such a sample should be largely free from terrestrial background, and using the exquisite sensitivity of modern mass spectrometry, the isotopic signature of ‘Little Boy’ should be unmistakable.44
| 3.2 |
Cesium and uranium in samples collected shortly after the bombing |
Since global fallout is rich in plutonium and in radioactive fission products such as 137Cs, soil samples that were protected from it should have great value for examining the fallout from the Hiroshima event alone. Two studies on soil, rock, and roof tile samples that were preserved in 1945 in Hiroshima itself, and which were retrieved from storage several decades later, exhibited distinct yet very low 137Cs activity [59,60]. The latter study actually reexamined a series of samples which were reportedly collected by famed nuclear physicist Yoshio Nishina on his visit to Hiroshima only three days after the bombing. Among these samples, the spread in activity is very large. The two samples that had been collected the closest to the hypocenter gave no detectable 137Cs activity. A single sample—obtained from the Koi area, which is located approximately 2 km from the hypocenter and is considered the zone most affected by fallout within the city limits—gave a value of 10.6 mBq/g; all other samples contained less than 1 mBq/g.
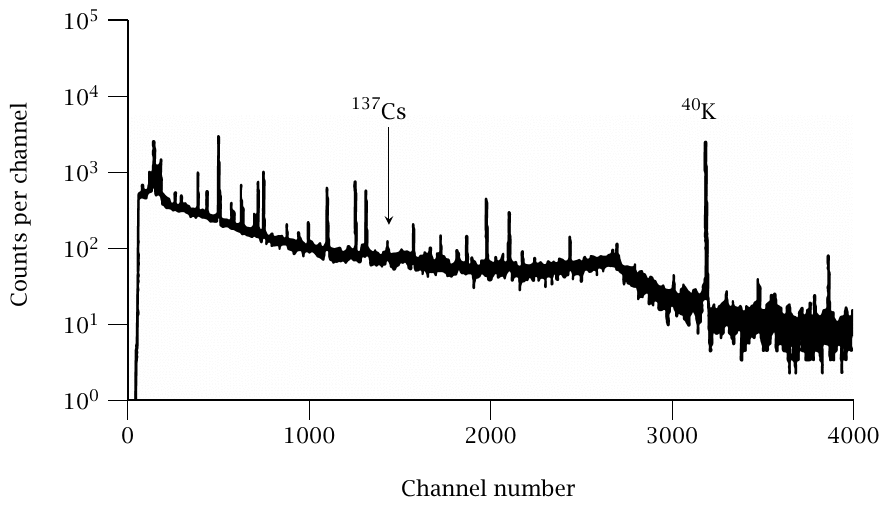
Figure 3.3 shows the γ-ray spectrum of one of the samples; the 137Cs peak is indicated. Since the measurement was reported in 1996, approximately two thirds of the 137Cs had decayed since the bombing. Most other peaks in the spectrum, particularly 40K, are caused by natural background radioactivity. Concerning this background, Shizuma et al. [60] note:
In 1950, soil samples were repacked in air-tight glass vials. … In the present measurement, soil samples were repacked in plastic containers … to eliminate the 40K gamma-ray background from the vial itself.
Let that sink in for a moment—the radioactivity of fallout from ‘Little Boy’, collected in the city three days after the bombing, is obscured by that of the glass vials used to preserve it.
Nishina’s samples have also been analyzed for uranium isotopes [61]. In this study, the isotope ratio 234U /238U was somewhat variable but always close to 1, whereas the abundance of 235U was consistent with natural background. Therefore, these soil samples, which are untainted by global fallout and very likely were not exposed to rain other than the black rain which transported the fallout,45 fit into the general pattern of detectable but very low levels of 137Cs, and negligible or absent bomb-derived 235U.
| 3.3 |
Cesium and plutonium in soil samples from the Hiroshima fallout area |
Yamamoto et al. [63] collected samples from soil underneath houses that had been erected throughout the black rain area after the Hiroshima bombing, but before 1950, and thus before most of the global fallout struck. All samples contained some 137Cs. The levels scattered by almost two orders of magnitude; however, even the highest values, which were observed in samples from two houses built as early as 1946, remained well below those which are caused in unprotected soil near Hiroshima by the subsequent global fallout. Thus, even in the black rain area, the 137Cs fallout from the Hiroshima bombing was small.
To explain the variability of their observed 137Cs levels, the authors quite plausibly invoke the excavation that may have occurred in preparation for construction in some of the buildings; however, they also state that
according to carpenters we interviewed, most of the wooden houses built around this time were built without causing major disturbance of the surface soil,
which suggests that the fallout was indeed quite inhomogeneously distributed within what is considered the fallout area.
We will revisit the question how the fallout may have come to be distributed so unevenly in Section 13.1.5. Here, we only need to note the following crucial point: whether or not the soil was disturbed before construction, it should have been protected from any fallout once the houses had been completed. It is therefore remarkable that, in all of Yamamoto’s presumably protected sub-floor samples, plutonium is also found.
Since the Hiroshima bomb is supposed to have consisted of enriched uranium, but not plutonium, its fallout should have contained at most minuscule amounts of plutonium.46 The observed activity of plutonium (239Pu +240Pu) activity was indeed only about 4% of that of 137Cs (see Figure 3.4B). However, after accounting for the much longer half-lives of both plutonium isotopes, its molar amount—that is, the total number of its atoms—exceeds that of 137Cs about 20-25 times on average.
A further consideration is the time of measurement. Plutonium has not decayed significantly since the bombing, but 137Cs decays much faster and would have been reduced to about one fifth of the original amount between the event and the publication of Yamamoto’s study; therefore, the ratio of abundance (Pu/Cs) at the time of the bombing would have been close to 4.
The authors, starting from the pious assumption that the official story of the bomb is true, stipulate that essentially no plutonium should have been present in pristine samples, and they ascribe that which they find to contamination by the global fallout. Since this completely voids the very premise of their study—namely, that their samples should be free of such pollution—one would expect some effort on their part to explain this unexpected outcome. However, no such explanation is forthcoming. More importantly, the authors do not test their assumption that such contamination was possible, which they could have easily done by obtaining soil samples from underneath houses built in the same area before August 1945. If the original premise of the study held, such samples should have been protected from any fallout; on the other hand, according to the authors’ revised hypothesis, fallout radioactivity should be present in all of these samples as well.
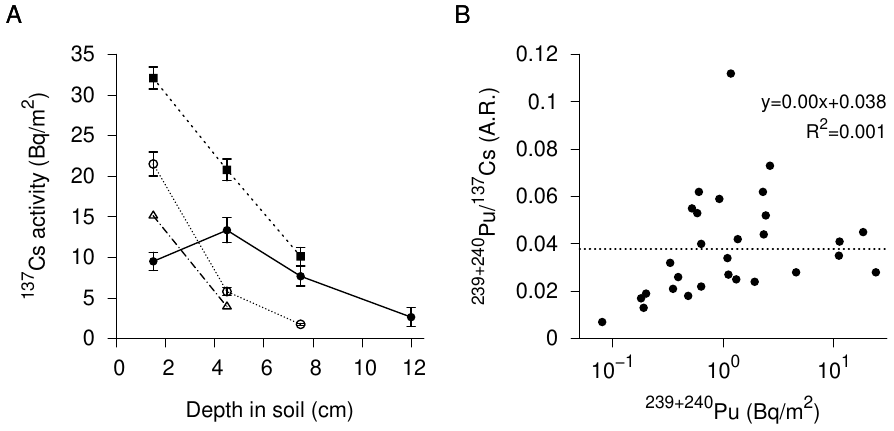
The only carrier I can think of that might transport some global fallout from soil outside a house to underneath it would be percolating rainwater. Note, however, that according to a preliminary report by the same authors [64] most of the radioactivity was found in a very shallow layer at the very top within the soil (Figure 3.4A). It is difficult to see how percolating water from outside the house would have produced such a distribution. Moreover, plutonium and cesium are not equally mobile within the soil; the aforementioned study by Sakaguchi et al. [56] shows that plutonium is carried downward faster than is cesium, and thus is more mobile. Hence, if indeed global fallout had been carried by percolating rainwater from soil outside to that underneath the house, the Pu/Cs ratio in the latter place should have been considerably increased. In this case, those among Yamamoto’s samples which contain the highest plutonium activity, that is, presumably the highest contamination, should also have the highest ratio of plutonium to cesium activity. However, if we plot the ratio of plutonium activity to cesium activity against plutonium activity, then no such trend is apparent, but the scatter is very large (Figure 3.4B). Thus, percolating rainwater can be dismissed as a mechanism for the presumed contamination.
There is, of course, another explanation for the plutonium in samples that should not have been touched by global fallout—namely, that they were indeed not touched by it, and the plutonium was really contained in the fallout of the Hiroshima bombing. This hypothesis has the dual advantage of simplicity and physical plausibility; its only difficulty is that it runs counter to the official narrative.
| 3.4 |
Variability of isotope ratios in the Hiroshima fallout |
Figure 3.4B showed that the ratio of 137Cs to plutonium (239Pu + 240Pu) in the fallout of the Hiroshima bombing is subject to large variation. A very considerable variation is also reported by Shizuma et al. [6] in the ratio between 137Cs and bomb-derived 235U among the black rain samples taken from a single piece of plasterboard (see Figure 1.2). This isotope ratio should be proportional to the bomb’s fission yield, which the authors peg at 1.2%; and according to their own calculations, the observed values of this ratio span a range of 0.62 to 8.1 times that yield. Similarly, the soil samples studied by Takada et al. [57], which were discussed in Section 3.1, show no clear correlation between the degree of 234U enrichment to 137Cs levels (Figure 3.5A).
In order to explain the marked variability in their observed isotope ratios, Shizuma et al. suggest that cesium and uranium were separated while being suspended in the air through “condensation,” but they do not provide any details on this proposed mechanism. They also do not discuss the possibility that heterogeneous isotope abundance ratios would result from the detonation directly and persist until after the expansion stage.
In this context, it is noteworthy that the samples studied by Yamamoto et al. [63] show a substantial variation in the ratio of 240Pu to 239Pu (Figure 3.5B). If we suspend disbelief for a moment and assume that both plutonium isotopes indeed originated from a nuclear detonation, their variable ratio could not possibly be due to differential condensation during transport, since any such effect would have to be based on different chemical properties of the elements in question; thus, we could not expect it to separate different isotopes of the same element (and Shizuma et al. do not suggest that it does). We would therefore have to ascribe the observed variability of the 240Pu /239Pu ratio to inhomogeneity of the detonation itself.
We had seen earlier that 239Pu arises from 238U through the capture of a single neutron, whereas the formation of 240Pu involves the successive capture of two neutrons, which must derive from separate fission events. We should therefore expect the proportion of 240Pu to show a positive correlation with the ratio of fission events to total plutonium, but this is not observed (Figure 3.5B). Thus, the hypothetical condensation mechanism proposed by Shizuma et al. would have to account for the loss of a correlation not only between 235U and 137Cs, but also between 240Pu and 137Cs (which represents the number of fissioned nuclei).
How plausible is this hypothetical separation mechanism anyway? I have not seen this question addressed in the scientific literature; therefore, I will give my own reasoning. I assume that immediately after a nuclear detonation each of the resulting nuclides will be present in multiple states of ionization. It is the net charge of each ion which should dominate its interactions with other particles, rather than the chemical reactivity in the neutral state of the chemical element to which the ion belongs. This applies in particular to its association with water molecules, which will begin once the temperature has dropped sufficiently.
As soon as some of the ions have managed to attract and retain a hydration shell, the resulting aerosol particles will scavenge additional ions in their path, and they will ultimately coalesce into larger droplets. Both of these processes will tend to mix different nuclides, not to separate them. Overall, differential condensation seems ill-suited to explain the very pronounced variations in isotope ratios between the individual large black rain droplets whose residues were studied by Shizuma et al. [6].
| 3.5 |
Cesium and plutonium in sediments from the Nishiyama reservoir near Nagasaki |
Since the Nagasaki bomb (‘Fat Man’) used 239Pu, as did most nuclear bombs tested in the subsequent decades, isotopic signatures are less suitable for distinguishing local from global fallout in this case. However, there is one circumstance that makes up for it: at Nagasaki, the heaviest fallout reportedly occurred in and around the Nishiyama reservoir, a small body of water located some 3 km from the hypocenter. The timeline of fallout deposition was examined by Saito-Kokubu et al. [65], who analyzed the sediments at the bottom of this reservoir. The lowermost peaks of plutonium and cesium were found at a depth of 435-440 cm (Figure 3.6A); these must represent the earliest fallout.
The entire sediment core contains only a single layer of macroscopic charcoal particles, which the authors quite plausibly ascribe to the deposition of soot from the burning city. Intriguingly, however, this layer is found at approximately 450 cm. Since the study was published 63 years after the bombings, sedimentation had occurred with an average rate of close to 7 cm per year; assuming this rate to have been fairly uniform, a separation by 10-15 cm corresponds to a time interval of approximately two years.
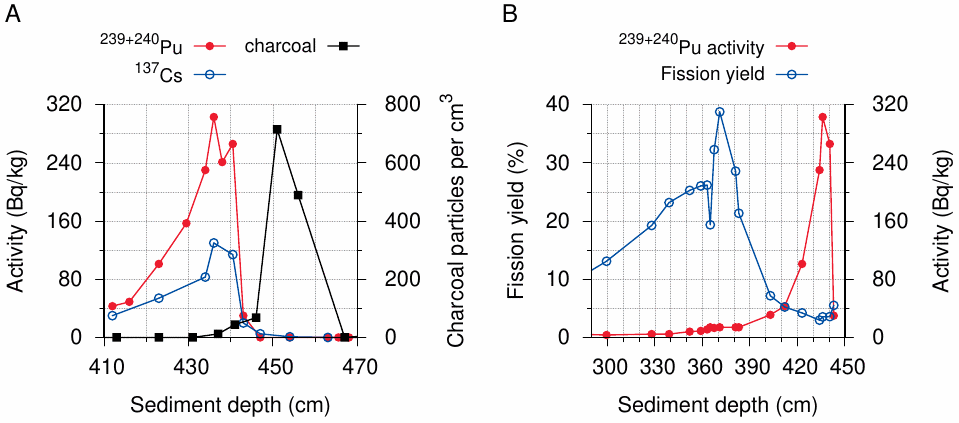
The authors of the study acknowledge that the peaks are separated, but nevertheless ascribe the radioactivity to the Nagasaki bomb fallout. They do, however, not provide an explanation for the mechanism of separation beyond stating that it requires ‘further study’. Considering the (macroscopic) size of the charcoal particles, we can assume that they are immobile within the sediment; thus, any separation would have to come about through upward migration of the radioactive isotopes. Such a migration, however, is very unlikely to have happened, for the following reasons:
- It lacks a driving force. On dry land, isotopes may slowly be transported downward through the soil by percolating water; however, considering that the reservoir is already water-filled, there will be no upward movement of more water into it from the ground underneath.
- The plutonium and cesium peaks are close to the charcoal layer, but they have practically no overlap with it. If the radioactivity had slowly leached out of the charcoal layer, then the radioactive peaks should be broader and exhibit more overlap with the charcoal layer.
- The findings reported by Sakaguchi et al. [56] show that plutonium is carried by percolating water more rapidly than is cesium; therefore, in the reservoir, the plutonium peak should have moved upward further than the cesium peak. However, the peaks of the two isotopes coincide.
Another incongruity emerges if we examine the ratio of plutonium to cesium in the sediments. Using the half-lives of the three isotopes (239Pu, 240Pu, and 137Cs), the age of a given layer of sediment, which can be estimated by interpolation from its depth, and the yield of 137Cs per fission event (approximately 6%), we can calculate the fission yield of the bombs whose fallout is contained in that layer. In Figure 3.6B, this calculated fission yield is plotted vs. sediment depth, along with the plutonium content. We see a low plateau of plutonium activity between 360 and 390 cm; in this region, which most likely contains the fallout from nuclear bomb tests conducted after the war, we see fission yields in the range of 20-40%. As we go deeper and reach the large peak of the supposed Nagasaki bomb, however, the fission yield drops to 5% and below.
According to standard lore [4], the Nagasaki bomb (‘Fat Man’) contained 6.2 kg of plutonium, of which 1 kg is said to have fissioned; this amounts to a fission yield of 16%. Thus, the fission yield of at most 5%, which is evident from the isotope ratio observed in the sediment layers said to contain the ‘Fat Man’s’ fallout, disagrees with the official narrative.47
As before, there is a politically incorrect but physically straightforward explanation for the observed discrepancies: charcoal and radioactivity are found in distinct layers of the sediment because they entered the reservoir at different times. The radioactivity was therefore not delivered by the ‘Fat Man’; this also accounts for the discordant isotope ratio, which is at odds with the bomb’s purported fission yield.
| 3.6 |
Enrichment of uranium to bomb grade: was it feasible in 1945? |
We have seen above that no highly enriched 235U can be demonstrated in the local fallout at Hiroshima, even though the bomb is said to have contained some 50 kg of it. We might therefore wonder if the technology for producing bomb-grade uranium even existed in 1945.
| 3.6.1 |
The state of the art according to Leslie Groves |
The overall leader of the ‘Manhattan Project’, General Leslie Groves, asserts that everything came together in the nick of time, with both plutonium and bomb-grade uranium becoming available in quantity just days before they were needed. There is, however, good reason to doubt his story.
The enrichment of 235U was carried out at Oak Ridge, Tennessee. According to Groves, three different plants were constructed for this purpose, each of which implemented a different physical principle of isotope separation. The first type was based on electromagnetic particle acceleration, the second on gaseous diffusion, and the final one on liquid thermal diffusion. In each case, construction was begun before the technical details of the process in question had been fully worked out. For example, with respect to the electromagnetic plant, Groves explains [40, p. 95 f]:
We then had to design, build and operate an extremely large plant with equipment of incredible complexity, without the benefit of any pilot plant or intermediate development: to save time we had early abandoned any idea of a pilot plant for this process. Always we were driven by the need to make haste. Consequently, research, development, construction and operation all had to be started and carried on simultaneously and without appreciable prior knowledge.
Anyone with some experience in real-world research and development will understand that the chance of success of such a venture will be infinitesimally small. Groves, of course, claims that this plant was highly successful, as were both of the others.48 To determine if this claim is credible, let’s put ourselves in Groves’ shoes and consider the following question: if our first isotope enrichment process is successful, will we scale it up, or will we gamble on a second process that has not yet been proven? If our first two processes work, will we scale up the more efficient one, or will we take our chances on a third?
Groves chose to gamble on a new process at both times, which of course suggests that neither of the first two processes worked as intended. Furthermore, he reports that the third plant was shuttered shortly after the war, indicating that it, too, was a failure.
| 3.6.2 |
The state of the art according to Klaus Fuchs |
In his book “Historical Dictionary of Atomic Espionage” [66], Glenmore Trenear-Harvey quotes from a conversation between the physicist Klaus Fuchs, a member of the Manhattan project and also a Soviet spy, with his spy handler Harry Gold from February 5th, 1944:
The work involves mainly separating the isotopes … should the diffusion method prove successful, it will be used as a preliminary step in the separation, with the final work being done by the electronic method. They hope to have the electronic method ready early in 1945 and the diffusion method in July 1945, but K [Fuchs] says that the latter estimate is optimistic.
Again according to Trenaer-Harvey, Fuchs met with another spy handler, Stepan Apresyan, in June 1944 and reported that
the ISLANDERS [British] and the TOWNSMEN [Americans] have finally fallen out as a result of the delay in research work on diffusion.
Fuchs continued working for the Soviets throughout the war and afterwards, but he never could give them a description of a viable enrichment process. This is apparent from the technical development pursued during the late 1940s and early 1950s by the Soviets themselves. The German physicist Max Steenbeck, who played a leading role in this effort, gives a first-hand account of it in his autobiography [67]. Before the experimental work began, the Soviets conducted broad consultations to identify the most promising physical principles of separation, and indeed there were some false starts before the successful development of the gas centrifuge. Thus, even though the Soviets had supposedly come into possession of America’s most prized atomic secrets, clearly those secret files did not tell them how to enrich 235U.
Steenbeck, who had himself been kidnapped by the Soviets as a civilian in Berlin, recruited several German and Austrian scientists and technicians from Soviet POW camps; two of them, Zippe and Scheffel, stayed and worked with him throughout his whole time in the Soviet Union. When finally all three men were allowed to return to Germany in the mid-1950s, Steenbeck joined his family at Jena in East Germany, whereas Zippe and Scheffel settled in the West. They were snapped up by Degussa, a metallurgical company with interests in nuclear fuel, for which they implemented the gas centrifugation technique on an industrial scale. Evidently, there was at the time no better or equally good process in place at this leading Western company. Centrifugation quickly superseded all other techniques for industrial 235U enrichment and remains the standard method today. Overall, this bit of history strongly suggests that the technology for enriching uranium to bomb grade, in quantity, did not exist in 1945.49
| 3.7 |
Did the first atomic test explosion really use a plutonium bomb? |
According to the official narrative, the “Trinity” test carried out on July 16, 1945 at Alamogordo (see Section 13.6.4) employed a plutonium bomb of the same type as the “Fat Man” bomb used on Nagasaki [40, p. 288]. However, this story is contradicted by some contemporaneous documents.
| 3.7.1 |
Arthur Compton in 1945: plutonium bomb several years away |
The ‘Interim Committee’ was a panel of leading scientists and politicians that was convened in 1945 to deliberate and advise on the future military and civilian use of atomic energy. Its affiliated scientific panel comprised leading physicists Robert Oppenheimer, Enrico Fermi, Arthur Compton, and Ernest Lawrence; all were present at the meeting in Washington, DC on May 31st, 1945. The following quote is taken from the protocol of this meeting [53]:
Dr. A. H. Compton explained the various stages of development. The first stage involved the separation of uranium 235. The second stage involved the use of ‘breeder’ piles to produce enriched materials from which plutonium or new types of uranium could be obtained. The first stage was being used to produce material for the present bomb while the second stage would produce atomic bombs with a tremendous increase in explosive power over those now in production. Production of enriched materials was now on the order of pounds or hundreds of pounds and it was contemplated that the scale of operations could be expanded sufficiently to produce many tons. While bombs produced from the products of the second stage had not yet been proven in actual operation, such bombs were considered scientific certainty. It was estimated that from January 1946 it would take one and one-half years to prove this second stage in view of certain technical and metallurgical difficulties, that it would take three years to get plutonium in volume, and that it would take perhaps six years for any competitor to catch up with us.
Apparently, the somewhat bland wording of this excerpt caused the bureaucrats who declassified this originally ‘top secret’ file to miss its true import; however, the meaning is unmistakable.
Compton’s first stage involves the isotopic enrichment of uranium. This comprises the production of both highly and weakly enriched 235U. The highly enriched uranium is for building bombs of the Hiroshima type; remarkably, the protocol claims that such bombs are “now in production.”
The second stage discussed by Compton concerns the production of plutonium. In this stage, he includes the generation of 239Pu within weakly enriched uranium by letting the latter go critical inside an atomic reactor (‘breeder pile’), as well as the subsequent purification of plutonium from the resulting complex mixture of uranium, 239Pu and 240Pu, and fission products.50
After these preliminaries, Compton discusses the prospects for the plutonium bomb. He states that the reactor-generated nuclide mixture is currently available on a scale of up to “pounds or hundreds of pounds.”51 However, this mixture will contain 239Pu only in proportion to the amount of 235U which was initially included in the pristine reactor fuel. In all likelihood, the ratio of 239Pu formed to 235U supplied was less than 1. Therefore, even ‘hundreds of pounds’ of the ‘enriched material’ would amount to several pounds of purified 239Pu at best.
Compton further states that, counting from the beginning of 1946, it will take an estimated 1.5 years to “prove this second stage in view of certain technical and metallurgical difficulties.” Since the first part of the second stage, namely, the production of ‘enriched material’, is already working, these difficulties must concern the purification of plutonium from it. Finally, he states that it will take yet more time to obtain plutonium ‘in volume’, which likely means in sufficient quantities for bomb manufacture. Even if we optimistically assume that ‘proving the second stage’ will already provide enough plutonium for a small number of bombs, Compton’s words still imply that, counting from the time of the meeting, two more years must pass before the first plutonium bomb can be assembled. Thus, the inference is unavoidable that a plutonium bomb could not possibly have been ready a mere six weeks later for the fabled test at Alamogordo, or for the bombing of Nagasaki three weeks after that—or even for the ‘Able’ and ‘Baker’ alleged nuclear bomb tests at the Bikini Atoll in 1946.
Considering the report’s surprising claim that 235U bombs are already in production as of May 1945, we may wonder why so much emphasis is placed on the plutonium bomb. The explanation may be in the expected explosive yields of various bomb types, which Oppenheimer states at this meeting as up to 20 kt for the 235U bomb, but up to 100 kt for the 239Pu bomb—and even 100,000 kt for the ‘third stage’, by which is meant the hydrogen bomb.
Overall, this remarkable protocol collides with two important aspects of mainstream atomic bomb lore—namely, that ‘Little Boy’ was the only 235U bomb available at the time, and that two 239Pu bombs would have been ready for use at Alamogordo and at Nagasaki. Should we take seriously its claim that 235U bombs “are now in production”? The small amount available of ‘enriched materials’ indicates that even reactor-grade uranium was still in short supply; that much more highly enriched 235U was available in the large quantities required for atomic bomb production is surely fiction.
We can assume that Leslie Groves and all of the scientists in attendance were aware of the true state of affairs. It thus seems that the fictional tale of uranium bombs already being in production was told at this meeting to keep some of the attending politicians and military officers in the dark. As among those present are listed Secretary of War Henry L. Stimson, James F. Byrnes, soon to be appointed as Secretary of State, and U.S. Army Chief of Staff George C. Marshall. However, even those participants who may have been deceived with respect to the uranium bombs must have understood soon after that the stories about the plutonium bombs having been detonated at Alamogordo and Nagasaki could not possibly be true. The protocol thus illuminates the striking extent of duplicity and deception engaged in alike by scientists, politicians, and military officers.52
| 3.7.2 |
Robert Wilson’s last minute experiments on uranium fission |
The physicist Robert Wilson oversaw the experimental research division at Los Alamos. In a paper which was published 1947 in Physical Review, he describes a very ingenious experiment to observe the lag time between the capture of a neutron by a 235U nucleus and the fission of that nucleus [69].
The manuscript of Wilson’s published report had originally been confidential, but it was declassified in 1956. While the two texts are largely congruent, the following intriguing statement is found only in the declassified manuscript [70]:
This sentence implies that even very shortly before the first atomic bomb test was carried out, a rather fundamental property of nuclear fission that might prevent the bomb from going off had not yet been established. It is of course hardly credible that the production of uranium bombs would have been begun, as the Interim Committee protocol asserts it had been, without this crucial experimental result in hand.
More pertinent in the current context, however, is the simple observation that Wilson’s last minute studies concerned 235U instead of 239Pu. Apparently, he was under the impression that the imminent test explosion at Alamogordo would use a uranium rather than a plutonium bomb. While this agrees with Compton’s statements before the Interim Committee, it is at odds with official atomic tradition. This conflict must have been the reason for scrubbing the quoted sentence from the manuscript before its publication in 1947.53
| 3.8 |
Conclusion |
Studies from neither Hiroshima nor Nagasaki furnish any clear evidence of radioactive fallout commensurate with the purported nuclear detonations. Levels of plutonium and 137Cs near Nagasaki are suitably high, but they do not agree with the bomb’s stated fission yield. Moreover, they were apparently deposited approximately two years afterward, which corresponds well with Compton’s estimated time of plutonium availability. The studies on the fallout of the Hiroshima bomb can be summed up as follows:
- No evidence exists of highly enriched 235U in the fallout. The measurements on soil samples indicate a very low degree of isotopic enrichment only, and those on black rain drops dried in situ suggest the same. A high degree of enrichment is only ever stipulated, and the calculations based on this premise result in vanishingly small absolute amounts of bomb uranium.
- 137Cs attributable to the Hiroshima bomb is readily detected. Its level remains well below the global fallout that arose from later bomb tests, but in most of the samples described in sufficient detail it nevertheless exceeds the amount we should expect from 235U measurements in conjunction with the key tenets of the official story of the bomb.
- Samples protected from global fallout also contain plutonium, in amounts and isotopic compositions that are incompatible with its formation by a detonating 235U bomb.
While none of these observations fit the ‘Little Boy’ narrative, all of them are consistent with the dispersal of reactor waste, for example by means of a ‘dirty bomb’. We also note that measured isotope ratios are highly variable, suggesting the use of several different batches of radioactive waste, within which the weakly enriched 235U had undergone fission to different degrees.
Overall, the findings and writings reviewed in this chapter consistently indicate that neither uranium nor plutonium were available in the required amounts and purities at the time of the alleged bombings, and that no atomic bombs were detonated. They also demonstrate inadequate, but determined efforts to forge the fallout of true nuclear detonations in both cities. With that in mind, let us now consider some of the physical studies adduced to prove that those nuclear bombings did indeed occur.