| 7 |
Sulfur mustard and napalm |
This chapter describes the chemical properties and biological effects of sulfur mustard, drawing on case reports from its uses in warfare, particularly in World War I. The chapter touches only briefly on the ‘nuclear’ bombings; its main purpose is to provide background for the discussion of clinical observations in the bombing victims in subsequent chapters.
The chapter concludes with an overview of the technical and medical aspects of napalm and its use in warfare.
Sulfur mustard is a synthetic poison that gained notoriety as the ‘king of battle gases’ in World War I, in which it caused more casualties than all other poisonous gases combined, even though it was first used only in 1917. Other battle gases like chlorine and phosgene had been used for longer, but their effectiveness had diminished because of protective measures, in particular gas masks. Sulfur mustard bypassed this protection because it attacks the skin, its fumes easily penetrating clothes and sticking to them. By damaging the deeper layers of the epidermis, it causes the formation of blisters, which may become confluent and cause the skin to peel off in large sheets. Agents of this kind are called vesicants; the term derives from the Latin word vesica (blister). Victims that are not protected by gas masks will also inhale the gas and suffer damage to the airways; in addition, sulfur mustard may be swallowed and then attack the intestinal tract.
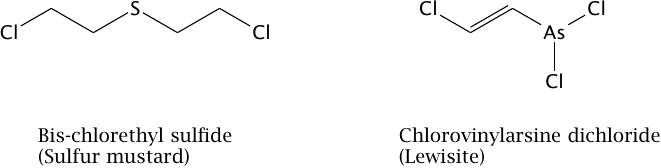
The second most important vesicant is lewisite; it, too, was developed during World War I, but apparently was not deployed. In World War II, both agents were stockpiled by several of the participants, but the only acknowledged use was by Japan in its Chinese campaign. According to Infield [105, p. 187], the U.S. had filled mustard gas into various types of aerial bombs, which were otherwise used for incendiaries; thus, sulfur mustard would have been ready and available for aerial attacks. In the 1980s, sulfur mustard was again used by Iraq in its war on Iran, and its most recent use reportedly occurred in the Syrian civil war [106].
While sulfur mustard and lewisite differ in chemical composition (Figure 7.1), their acute toxic manifestations are similar [21].83 For reasons detailed below, we consider sulfur mustard the most likely vesicant to have been used in Hiroshima and Nagasaki, and we will therefore focus on this agent.
| 7.1 |
Physicochemical properties |
Sulfur mustard has a boiling point of 217∘C [35] and a melting point of 14∘C; for deployment at cooler temperatures, the melting point can be lowered by mixing the poison with organic solvents. In its pure form, liquid sulfur mustard is oily and poorly water miscible, which slows down its hydrolysis (decomposition by reaction with water). Slow decomposition, a tendency to penetrate porous materials such as wood or bricks, and its high boiling point allow it to persist in the environment for potentially long periods of time. This is illustrated by these words of British World War I veteran Cecil Withers, quoted from Fitzgerald [104]:
I suffer badly from phlegm and from coughs and colds a lot. That all started when the British were shelling hard at the last Battle of the Somme. One of the shells disturbed the residue of mustard gas that had been lying there for months. They talk about secondary smoking … I got secondary gas.
In contrast to sulfur mustard, lewisite has a low boiling point (77∘C) and thus is much more volatile; it is therefore likely to dissipate much more readily. We know that the noxious agent used in Hiroshima persisted for weeks [16,34]; this is the first reason to suspect the use of sulfur mustard rather than lewisite. Another reason is the foul smell, which in Hiroshima was noted by many [15,16]. Apparently, this smell arises mostly from contaminants in the technical product, which are numerous [108]; the pure product has only a faint smell [109, p. 32]. Lewisite, in contrast, is said to smell only slightly of geraniums [110].
| 7.2 |
Mode of action and toxicokinetics |
The molecular structures of sulfur mustard and of lewisite are shown in Figure 7.1. Evidently, they are quite different; in particular, the two chloroethyl groups of the mustard molecule, which mediate its reaction with DNA (see below) are lacking in lewisite. This suggests that their reactions with molecules within the cells will be different, too, even though the consequences may be similar.
| 7.2.1 |
Reaction with DNA |
The reaction of sulfur mustard with DNA begins with the formation of an episulfonium ion (Figure 7.2). This three-membered ring is highly unstable and may react with any nucleophiles within the cell; but, for the same reasons as with ionizing radiation (Section 2.11), the most consequential target molecule is DNA. Any of the four bases found in DNA84 may react, but the most reactive one is guanine, and in particular the specific nitrogen (N7) in the imidazole ring shown in the Figure. Importantly, sulfur mustard is a bivalent molecule; both of the two chloroethyl (–CH2–CH2–Cl) groups attached to the central sulfur atom can react in the same manner. This may cause the formation of a cross-link between two bases on opposite strands of the DNA molecule; and downstream of such cross-links, both strands may break,85 resulting in the same kind of lesion also observed with ionizing radiation. An important role of such cross-links in the biological effect of sulfur mustard is supported by the early finding that similar compounds in which one of the two reactive groups is missing have much lower toxicity [109, p. 35].
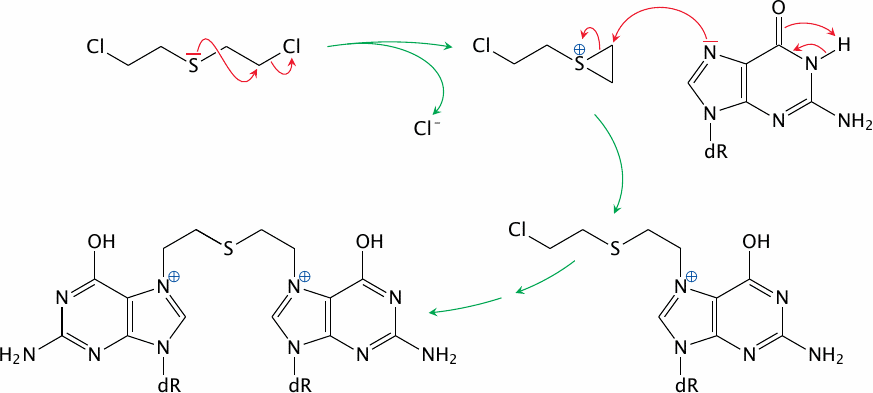
The similarity of the mutagenic DNA lesions caused by ionizing radiation and by sulfur mustard explains that the two noxious agents produce similar biological effects both in the short term, such as bone marrow damage and epilation, and in the long term, such as leukemia and cancer. The reactivity of lewisite toward DNA has received surprisingly little attention; unlike sulfur mustard, however, lewisite has no clearly documented mutagenic or carcinogenic potential [21,111]. The significantly increased incidence of leukemia and of some solid tumors among survivors of the Hiroshima and Nagasaki bombings [112,113] thus further supports the thesis that sulfur mustard rather than lewisite was used in the attacks on both cities.
| 7.2.2 |
Depletion of glutathione |
While reaction with DNA mediates most of the damage at low concentrations of sulfur mustard, reactions with other nucleophiles provide an alternate mechanism of toxicity at higher levels. A particularly important molecule is glutathione, which has a key role in scavenging various kinds of toxic compounds inside the cell. If glutathione is depleted by its reaction with sulfur mustard, this will impair the cell’s ability to neutralize reactive oxygen species (ROS), which arise as the main products or as side products of many metabolic processes; the unscavenged ROS may then cause cytotoxic effects [114].
One biochemical pathway that involves ROS is the formation of skin pigment (melanin); and the melanocytes (pigmented cells) of the skin, which carry out this pathway, are more susceptible to sulfur mustard toxicity than are the non-pigmented keratinocytes [115]. Accordingly, levels of exposure that kill the melanocytes yet permit the keratinocytes to regenerate may cause skin depigmentation. On the other hand, lower levels of sulfur mustard that permit both keratinocytes and melanocytes to regenerate may result in hyperpigmented skin areas. The latter are often seen delineating the depigmented ones.
Glutathione reacts with sulfur mustard via its sulfhydryl (–SH) group, which makes an excellent nucleophile for attacking the episulfonium intermediate shown in Figure 7.2. Although the chemistry is different, sulfhydryl groups also react strongly with lewisite; this suggests that the similarity of the early manifestations on skin and mucous membranes is indeed due to this mechanism. Experimental data on the reaction products formed by lewisite in vivo are, however, very sparse [21,111].
| 7.2.3 |
Systemic uptake and distribution |
Sulfur mustard is taken up through skin contact, inhalation, and ingestion. Soldiers exposed to sulfur mustard in World War I, as well as the workers in the factories producing the poison, were often protected by gas masks; aware of the danger, they would mostly have avoided ingestion of contaminated food or water. In contrast, the unprotected and unaware victims in Hiroshima and Nagasaki most likely took up significant amounts by all three routes.
When applied experimentally to the skin of experimental animals, 80% of the compound will typically evaporate, but the other 20% will be taken up. Approximately 80% of that latter fraction, or 16% of the total, will indeed reach the blood circulation and then the inner organs, while the remainder (4% of the total) will react and remain within the skin itself [116]. The fraction taken up into the system distributes between different organs. While the relative abundances found in different organs vary somewhat between studies that use different methods of detection—chemical [117], radioactive tracers [118,119], or DNA damage [120]—it is apparent that organs with strong blood flow receive and retain the highest amounts. These organs include the brain, the lungs, the spleen, and the kidneys.
As noted earlier, sulfur mustard is poorly water-miscible. Such substances are called hydrophobic or lipophilic, and they tend to accumulate in tissues which a high content of of lipids, i.e. fat-like substances. The brain is not only strongly perfused, but also particularly rich in lipids in the form of myelin, which enwraps many nerve fibers, endowing them with low membrane capacitance and high speed of conduction. It is therefore understandable that Batal et al. [120] found the highest abundance of DNA adducts in the brain, slightly ahead of the lungs. However, since cell proliferation in the brain is generally very slow, this organ is not very sensitive to the consequences of DNA damage by sulfur mustard; this parallels its relatively low susceptibility to radiation.
With the passage of time, sulfur mustard will redistribute from the brain and other highly perfused organs into the tissue with the highest fat content—fat tissue. This was demonstrated by Drasch et al. [117], who examined the body of an Iranian soldier who had succumbed to sulfur mustard poisoning one week after exposure. It is notable that the sulfur mustard observed after this time was still in its native, unreacted form. Slow redistribution, via the bloodstream, from fat tissue to other organs would likely give rise to protracted DNA and cell damage over time; this may contribute to the oft-noted slow recovery of sulfur mustard victims, and also to the delayed onset of ‘radiation sickness’ in patients from Hiroshima and Nagasaki (Section 8.8).
Yue et al. [121] compared the abundance of DNA adducts in several major organs after experimentally exposing rats to sulfur mustard. When normalized to the total amount of DNA in each tissue, the highest content was found in bone marrow, followed by the brain, pancreas, lungs, and spleen. The high susceptibility of the bone marrow to sulfur mustard is a long-established fact [122], as is that of the gonads. Nevertheless, we note that high levels are reported consistently in some organs—brain, lungs, and kidneys—that are among the least susceptible to ionizing radiation.
| 7.2.4 |
Metabolism |
The reactive nature of sulfur mustard makes it amenable to several pathways of metabolic conversion and inactivation. We already mentioned the reaction with glutathione; this reaction is facilitated by the enzyme glutathione-S-transferase, which is particularly abundant in the epithelial cells of the liver and the small intestine. Glutathione conjugation is an effective detoxification pathway for drugs and xenobiotics; as long as glutathione is not depleted by large amounts of substrate—such as, for example, sulfur mustard in the skin—this reaction is beneficial.
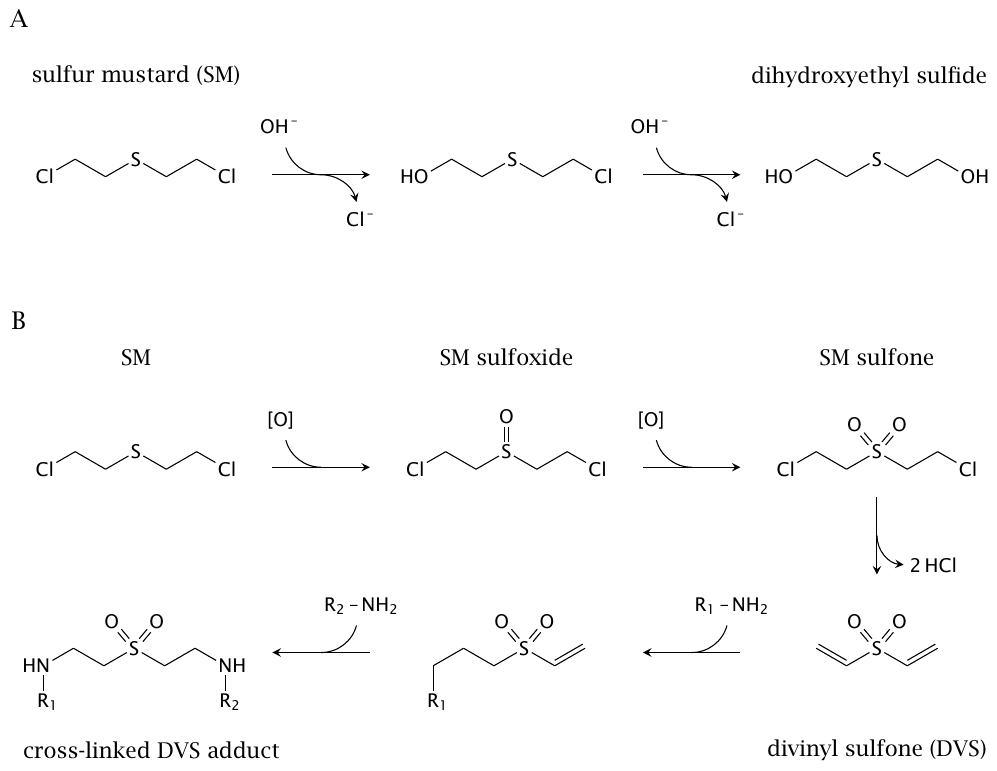
Sulfur mustard is also susceptible to hydrolysis, which occurs in two steps and results in its inactivation (Figure 7.3A).86 Another important reaction is oxidation, which occurs extensively in vivo[123]. The enzymes responsible have apparently not been characterized. Until such evidence becomes available, both cytochrome P450 and peroxidase enzymes are plausible candidates. The first oxidation intermediate is the sulfoxide, which has low toxic activity (Figure 7.3B); however, a second oxidation will give the sulfone, which can eliminate HCl and thereby turn into divinyl sulfone, a highly reactive and mutagenic compound [124]. In this context, it is noteworthy that a high level of peroxidase activity occurs in the thyroid gland. Thyroid peroxidase is known to mediate sulfoxidation of structurally similar thioether compounds [125], and conversion of sulfur mustard to divinyl sulfone in the thyroid gland might expose this organ to increased carcinogenic activity. Thyroid cancer has been observed in Iranian sulfur mustard victims [126], and its incidence is also significantly increased in Hiroshima and Nagasaki survivors [127].
| 7.3 |
Clinical and pathological manifestations |
From its biochemical mode of action, it is clear that sulfur mustard is not selective for any organ or cell type. Therefore, the severity of damage to any particular organ is largely governed by the extent of its exposure. Directly exposed are usually the skin, the eyes, and the airways and lungs. The fraction of the poison that is taken up systemically will preferentially affect organs that are strongly perfused, such as the lungs, the brain, the spleen, the kidneys, as well as the adrenal and thyroid glands. In organs exposed to high doses, glutathione depletion is more likely to cause damage in the short term; in those subjected to lower doses, the tendency to respond to DNA damage with apoptosis (programmed cell death) is a crucial determinant. The latter category includes in particular the gonads, the bone marrow, and the lymphatic tissues.
| 7.3.1 |
Blood circulation |
Most organs will become exposed to sulfur mustard through the blood circulation; and since the blood levels are evidently high enough to cause severe damage in many of these organs, we can also expect toxicity to the blood circulation itself.
In experimental animals exposed to sulfur mustard, the larger blood vessels (arteries and veins) were observed to lose tone and become dilated; the affected organs appeared congested, i.e. more strongly filled with blood than usual. The smallest blood vessels (the capillaries) became leaky; plasma fluid and proteins were lost from the bloodstream, as sometimes were blood cells, and caused the surrounding tissues to swell up [28]. Such findings explain the clinical picture of hypovolemic shock and general edema in severely exposed victims [128] and also in experimental animals [17].87 Leakiness of the microcirculation is also apparent from the loss of plasma proteins in the urine; and acidity of the urine indicates metabolic acidosis, which is a hallmark of severe circulatory shock [109, p. 228].88 The poisoned victims will initially look pale, as perfusion of the skin is largely shut off in favor of the vital organs. In later stages, they will appear swollen and cyanotic. The loss of plasma fluid should also trigger intense thirst; this is documented in cases of severe mustard gas poisoning [109, p. 228], and it is also observed in other diseases that cause generalized leakiness of the microcirculation, or capillary leak syndrome [131]. Even with intensive care readily available, this condition is often fatal [132], and such an outcome will of course be even more likely under field conditions.
The proteins contained in the extravasated plasma fluid include coagulation factors and fibrinogen, which will become activated and may solidify. Particularly in the lungs, this can result in the formation of fibrin ‘casts’ that obstruct the lumen of the bronchi and bronchioli, which has been observed both in autopsies of human victims [28,109] and in experimental animals [133].
| 7.3.2 |
Airways and lungs |
In mustard gas victims not protected by gas masks, the airways and lungs are prominently affected. The inhaled sulfur mustard will condense on the mucous membranes and attack the epithelial cells within them. The necrotic (dead) cell layers may remain in place, held together by extravasated and coagulated fibrin, as so-called pseudomembranes [134], or they may desquamate in a manner similar to the epidermis of the skin. Either way, the victims will experience hoarseness and pain in the throat and chest, and they will have difficulty breathing and swallowing.
The bronchi may become obstructed by fibrin cast formation (see above) or by clots forming from blood spilling out of damaged blood vessels [133]. Coagulation can also be activated within the lung’s blood vessels themselves; the clots formed in place will then block the further flow of blood through the lungs [135]. Since partially obstructed bronchi tend to let more air in than out, air will become trapped in the peripheral lung tissue, a condition known as emphysema [28]. Distended zones of lung tissue will then compress adjacent ones and disrupt their ventilation. Such collapsed areas of lung tissue are referred to as atelectases; they may also be caused directly by complete occlusion of the bronchi that ventilate them. Elevated pressure and structural injury may induce the trapped air to leave its regular confines and enter the interstitial space of the connective tissue; this is referred to as interstitial emphysema.
If the patient survives this initial stage, the injured lung tissue will be susceptible to infections, and thus foci of bronchopneumonia will develop. Overall, lungs damaged by sulfur mustard will exhibit general circulatory congestion and a varied pattern of bronchial obstruction, hemorrhage, and inflammation.
| 7.3.3 |
Eyes |
Affliction of the eyes is usually early and painful (Figure 7.4), but also transient. The lesions to the exposed parts of the eyeball, the cornea and the conjunctiva, are similar in principle to those found on the epidermis and mucous membranes, with necrosis and desquamation; however, they are mitigated by the prompt and steady rinsing action of the tear fluid.

The corneal epithelium, when damaged, will initially appear turbid and then erode; this causes impaired vision, pain and reflexive blepharospasm. In combination, these symptoms will create a subjective perception of blindness; Alexander [22] reports that some of his patients at Bari believed themselves permanently blinded until their eyes were forced open to prove to them that they could still see. The deeper layers of the cornea, and the remainder of the eyeball, may escape undamaged. The eroded epithelium will regenerate from the periphery toward the center. In most cases, the loss of vision is reversible within days or a few weeks.
While the above covers the consequences of external exposure, it is also necessary to consider the possible effects on the eyes of sulfur mustard transported in the bloodstream. While the literature offers no pertinent experimental evidence on sulfur mustard itself, some studies have been reported on various functionally similar compounds, including nitrogen mustard and busulfan, which are or were used in the treatment of cancers and leukemias. Patients thus treated may develop symptoms in parts of the eyeball not usually affected by superficial exposure. Uveitis, that is, inflammation of the iris and adjacent soft tissue structures, and edema of the retina have been described in patients receiving cancer treatment with nitrogen mustard [136]. Cataract, which afflicts the lens, has been induced with nitrogen mustard and busulfan in experimental animals [137,138]. Similar effects are likely to occur after systemic uptake of sulfur mustard. In addition, we can expect bleeding in the retina and other places in patients with generalized purpura due to bone marrow suppression (see Section 8.2.1).
| 7.3.4 |
Skin |
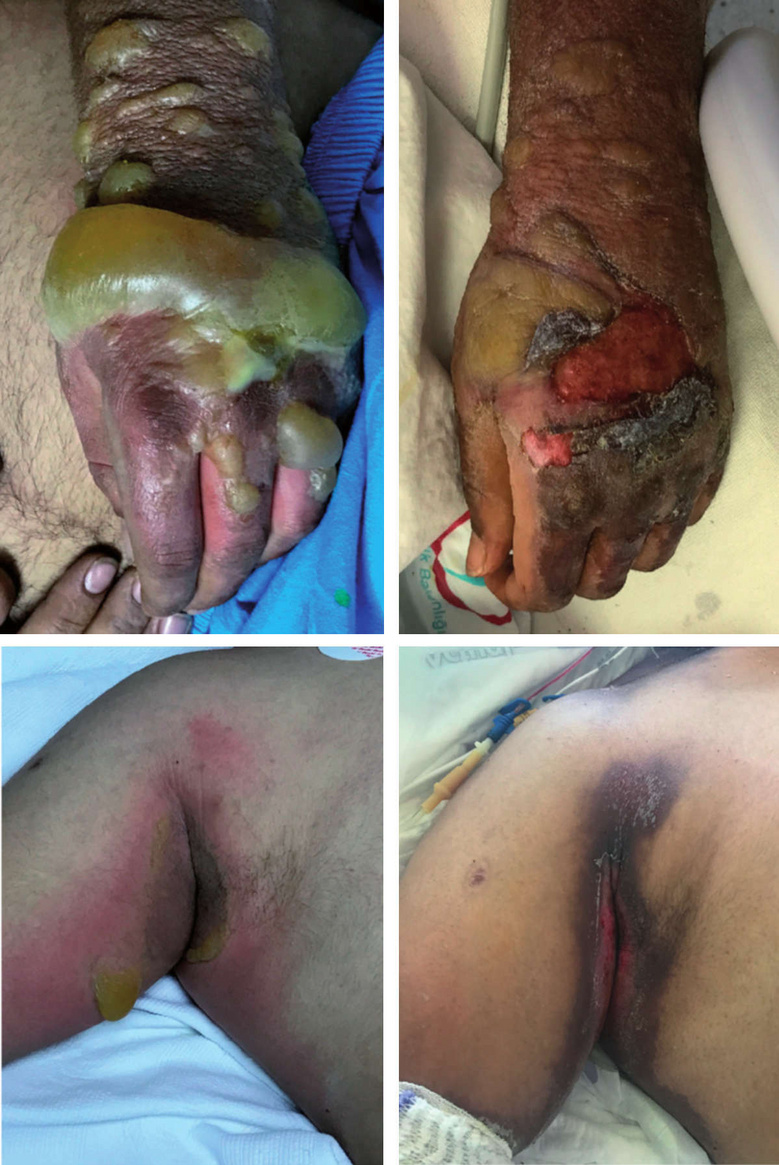
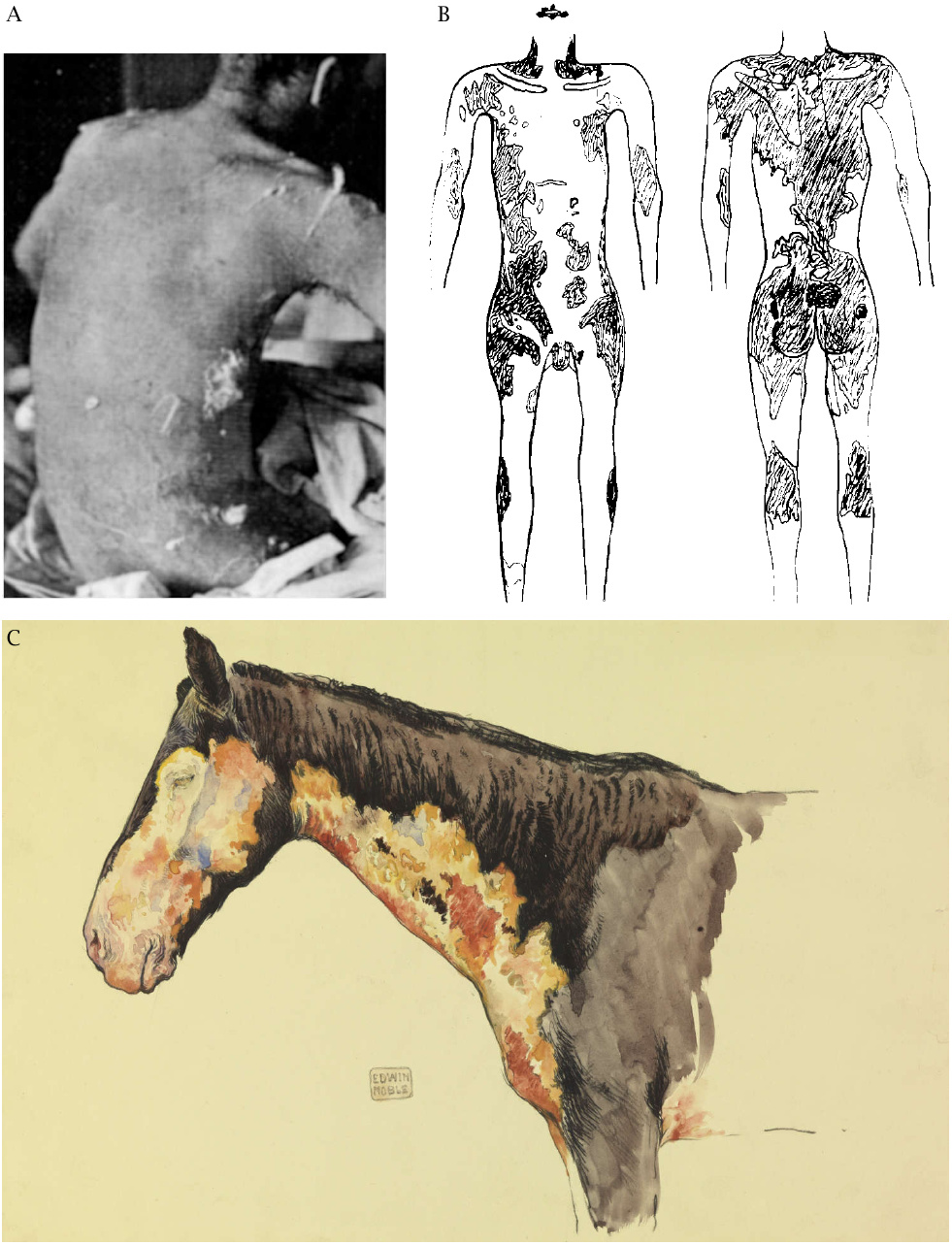
While skin blisters are a prominent feature of mustard gas lesions, the spectrum ranges from mere erythema over desquamation and blisters to deeper necroses of all layers of the skin and the underlying soft tissues. The severity will vary not only with the amount of sulfur mustard applied, but also with the texture of the skin and its humidity; the palms of the hand have thicker skin and are less susceptible, whereas areas covered by tender and humid skin such as the armpits and genitals are more so.89 Severe lesions may be surrounded by a halo of less severely afflicted areas. When such lesions heal, the more lightly affected peripheral areas tend to become hyperpigmented (Figure 7.5), whereas the more severely affected ones will show depigmentation. The underlying reason was discussed in Section 7.2.2 above.
The skin may be exposed by being splashed directly with liquid sulfur mustard, but also by indirect contact with contaminated weapons or other objects, as well as by the fumes, which easily penetrate clothes, even in multiple layers. While mustard splashed on exposed skin areas may be rapidly wiped and washed off before doing much damage, contaminated clothes may function as a reservoir of the poison and cause more severe damage to the skin underneath. Examples of skin lesions observed underneath clothing are shown in Figure 7.6. Similarly, Alexander [22] reports that, among the mustard gas victims at Bari, those who stripped off their contaminated clothes on their own initiative fared much better than those who kept them on for the night after the disaster. Such apparent negligence can be understood if we consider that the onset of mustard skin lesions is typically delayed by several hours; once the pain becomes perceptible, the poison has already been taken up, and the damage is done. On the time course of the clinical manifestations, the American military physician Harry Gilchrist notes [139, p. 44]:
At first the troops didn’t notice the gas and were not uncomfortable, but in the course of an hour or so, there was marked inflammation of their eyes. They vomited, and there was erythema of the skin. … Later there was severe blistering of the skin, especially where the uniform had been contaminated, and by the time the gassed cases reached the casualty clearing station, the men were virtually blind and had to be led about, each man holding on to the man in front with an orderly in the lead.90
A careful experimental study on the time course of mustard skin lesions [140] also documents a slow, gradual progression. They early stage consists in a massive edema through extravasation, indicating capillary damage. Blood flow remains intact for several days, even though necrosis of the tissue is underway; vascular occlusion and sequestration of necrotic tissue finally occur after some 10 days. Such a time course resembles clinical observations.
| 7.3.5 |
Digestive tract |
The earliest and most common gastrointestinal symptom is vomiting. Unless it is bloody, however, vomiting need not be due to direct action of the poison on the digestive organs, but may instead result from its stimulating effect on the area postrema in the brainstem, which triggers vomiting in response to various chemical agents. A more specific indication of damage to the intestinal organs themselves is diarrhea, which in severe cases may also be bloody.
Warthin and Weller [109] relate that physicians who had been treating cases of mustard poisoning in World War I disagreed as to whether diarrhea constitutes an early and typical symptom of mustard gas poisoning. Two cases described in detail by Heitzmann [28] developed diarrhea only about 10 days after the exposure; on the other hand, Warthin and Weller [109, p. 75] describe an acute case with diarrhea setting in promptly, together with vomiting, and they also report rapid onset in experimental animals injected with the poison (pg. 91). Dacre and Goldman [17], too, cite a number of experimental animal studies and human case reports that list early diarrhea as a typical symptom of mustard gas poisoning.
If and when diarrhea occurs in a given case of mustard gas poisoning may simply depend on the dosage. The digestive tract may receive sulfur mustard both by ingestion and through the bloodstream. In the first case, one would expect higher local levels and earlier onset of symptoms, whereas in the latter case levels in the GI tract may be lower and the onset of manifest symptoms delayed, as is the case with the bone marrow.
Autopsy reports paint a somewhat variable picture, with edema, focal or regional necroses, pseudomembranes, hemorrhages within the mucous membranes or spilling out into the lumen, and secondary infections. Overall, the pathological features are rather similar to those observed in the respiratory tract.
| 7.3.6 |
Bone marrow, spleen, and gonads |
These organs host cell types which are highly susceptible to radiation, and which likewise are highly susceptible to the genotoxic effect of sulfur mustard. In many cases, it is indeed the bone marrow toxicity that causes the patient’s demise, through either uncontrollable bleeding due to lack of thrombocytes, or unmanageable infections due to the lack of leukocytes. Accordingly, in the autopsies of such patients, one finds a barren bone marrow, absent sperm cell production, and depletion of lymphocytes in the spleen. None of these observations distinguish organ damage by sulfur mustard from that caused by radiation.
| 7.3.7 |
Kidneys, liver, and brain |
In most cases, these organs show signs of damage to the vascular system rather than to the organ-specific epithelial or nerve cells. The blood vessels are congested, occasionally bleeding into the tissues has occurred; in the liver, there may be some signs of fatty degeneration, and in the kidneys protein may have seeped out of the blood vessels, into the urine-conducting and -processing conduits (the tubuli; [28]). These changes, while not overly dramatic, are not expected in patients exposed to doses of radiation that do not kill on very short notice (1-2 days).
| 7.4 |
Napalm |
The name “napalm” denotes gasoline-based incendiaries that have been rendered viscous and sticky using a variety of suitable additives. When filled into bomb shells and ignited by a detonating charge, usually with the help of white phosphorus, napalm will disperse in large burning gobs, which will adhere to the surfaces they strike. Since gasoline has a very high heat of combustion, the burning clumps of napalm will very effectively ignite flammable targets, and they will do extensive damage to non-flammable ones—including, of course, the human body.
One thickening additive that was found to be both cheap and effective is a combination of naphthenic acid with a mixture of fatty acids produced from coconut oil. The word “napalm” combines the names of naphthenic acid and of palmitic acid, the latter being one component of the coconut-derived mixture. These acids were converted to their aluminum salts, or soaps, before being combined with the gasoline.91 According to Björnerstedt et al. [141], this ‘proper’ napalm is particularly suitable for flamethrowers, whereas polymeric thickeners have been widely used when filling incendiary bombs.
While napalm strikes its human victims with severe injury and often death, the medical literature on its effects is astonishingly sparse. As of this writing (in 2019), a simple search for “napalm” on PubMed retrieves 29 articles, of which only 7 (seven) are written in English, and none of these provides much useful detail.92 The most substantial medical articles, albeit also low in number, have been contributed by military physicians from the former Soviet Union, which aided its allies North Korea and North Vietnam in the treatment of napalm victims during the respective wars [142–144]. Prominent findings reported by these physicians include:
- napalm burns tend to be very deep (3rd and 4th degree);93
- in the acute stage, loss of consciousness and circulatory shock are frequent;
- burns that affect the face or areas near it often damage the airways and lungs, leading to hypoxia and sometimes asphyxiation;
- burns to the face will often involve the eyes, with scarring of the eyelids causing secondary damage to the corneas;
- more than 35% of the North Korean soldiers who had been hit by napalm died on the spot;
- slightly more than half of all Korean survivors developed keloids, that is, hypertrophic, prominent, swollen scars.
According to Dolinin [143], the U.S. used approximately 200 tons of napalm per day during the Korean war, whereas during the Vietnam war daily production—presumably similar to daily use—amounted to about 700 tons. Much of it was, of course, used against civilians. Only occasionally has the American and international public been confronted with the resulting horrors; awareness seems to be limited to the iconic ‘Napalm Girl’ Kim Phuc (see Figure 9.5). It is quite difficult to find images of any other Vietnamese napalm victims, but some are shown in William Pepper’s 1967 article “The Children of Vietnam” [145] in Ramparts magazine, which as of this writing is available online. Several of these victims are very severely disfigured. Images of acknowledged Japanese napalm victims—other than scorched and shriveled corpses left behind by the Tokyo bombing of March 1945—seem likewise to have been purged from the public record.